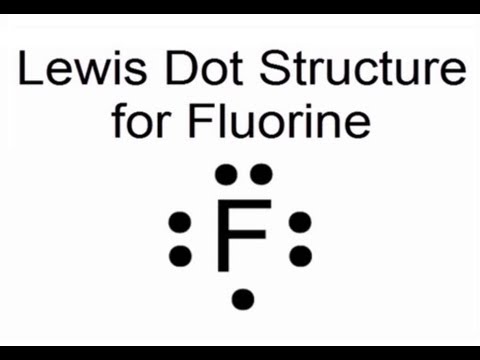
Electron Dot Structures – Helpful tools in thinking about bonding. Pictorial Electron dot structure – valence electrons are represented by dots placed around the. Electron Dot Structures – Helpful tools in thinking about bonding.
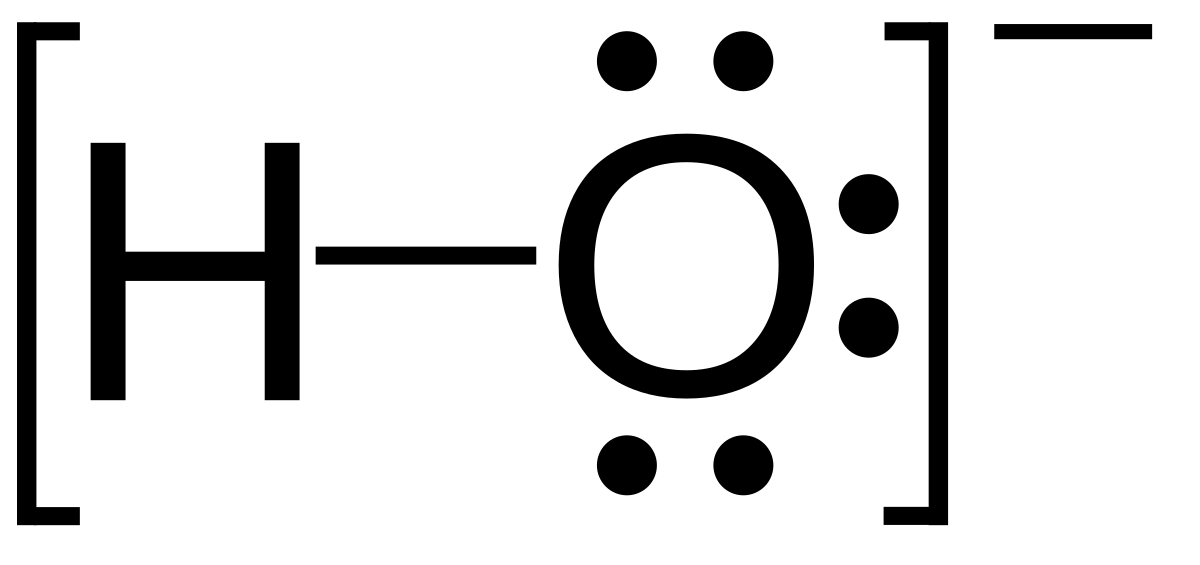
Pictorial Electron dot structure – valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a monatomic ion.
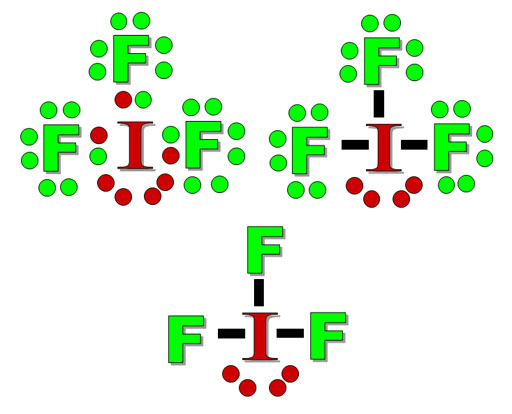
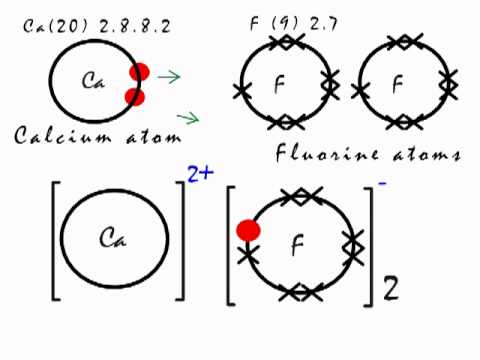
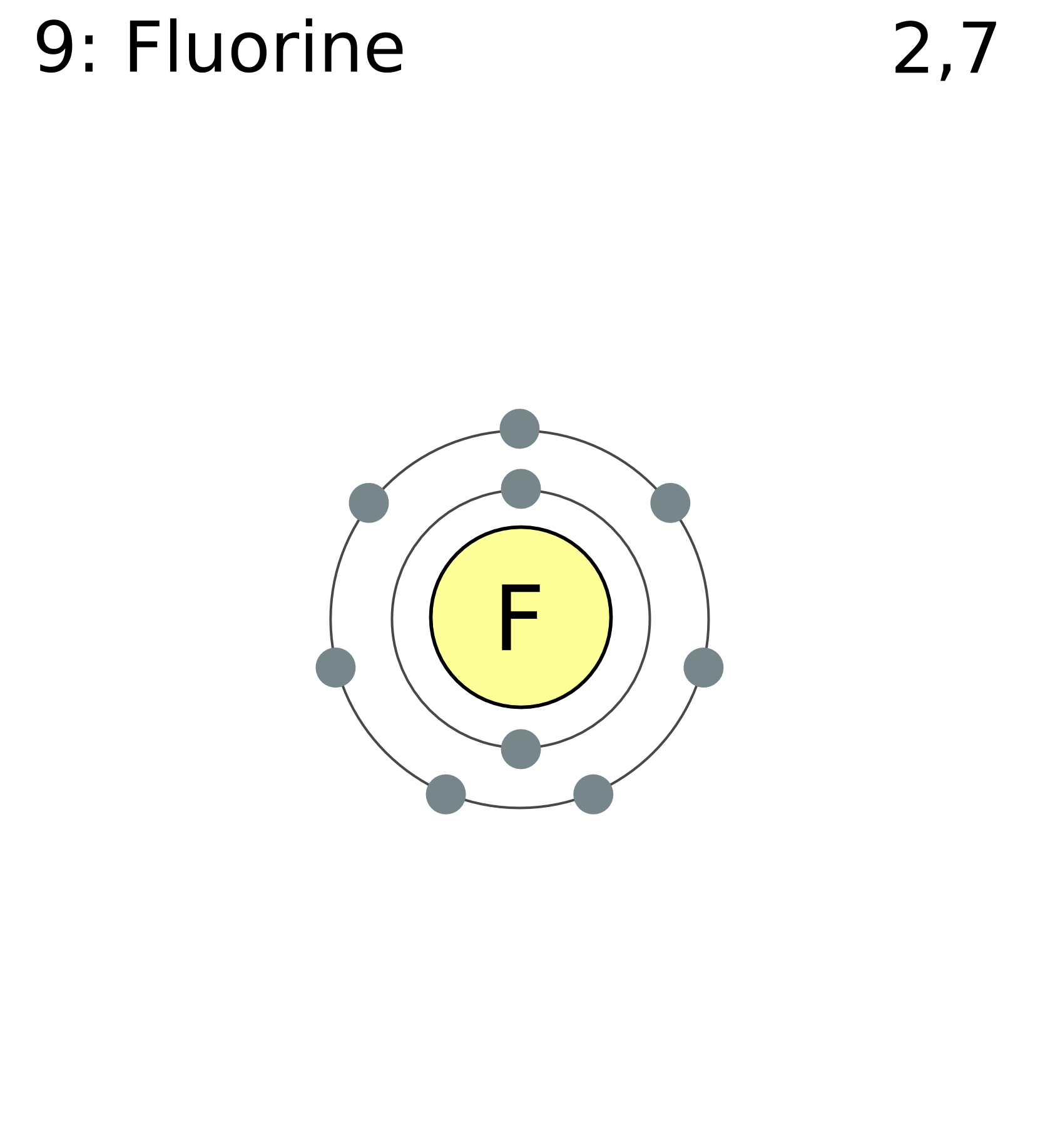
In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron.
Lithium is in Group 1 of the Periodic Table. Fluorine.
Structure of a. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure Choose the statement the correctly identifies the most stable of the elements. a.
lithium b. carbon c.
fluorine d. neon.
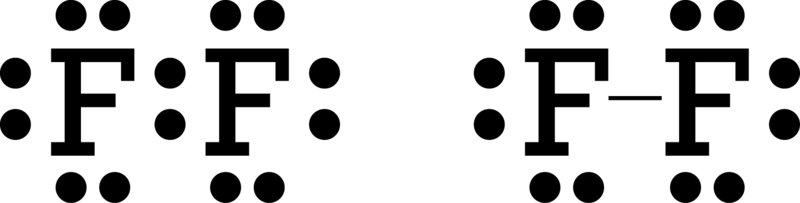
A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. The Lewis structure of a positive ion (cation) is positioned adjacent to the Lewis structure of a negative ion (anion).
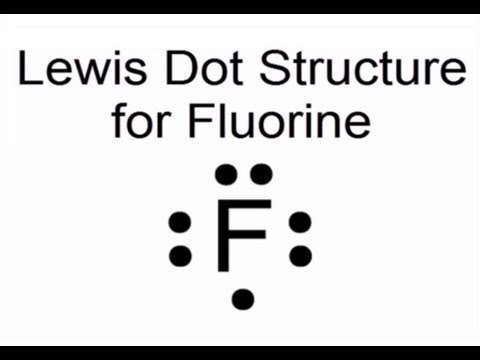
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
The Lewis structure was named after Gilbert N. Lewis, who introduced it in his article The Atom and the Molecule. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.What is the Lewis electron-dot diagram for a fluoride ion? | SocraticLewis Structures Chemistry Tutorial