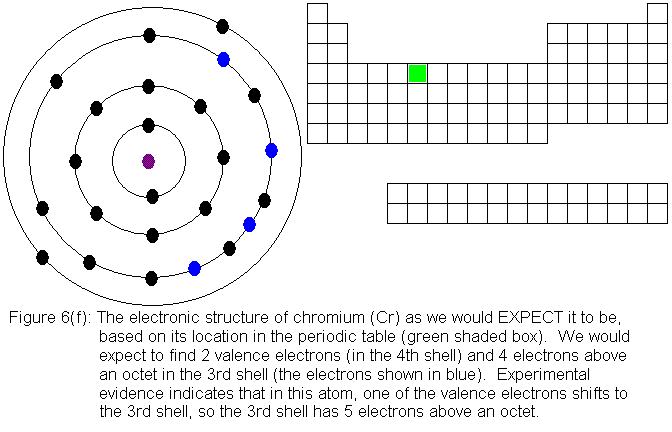
Structure, properties, spectra, suppliers and links for: Chromium(III) ion, Comprehensive information for the element Chromium – Cr is provided by this page including scores of Atomic Structure of Chromium Electron Dot Model. When the electrons are filling up the energy levels, both the 3d shell and the 4s shell have one electron in their orbitals.
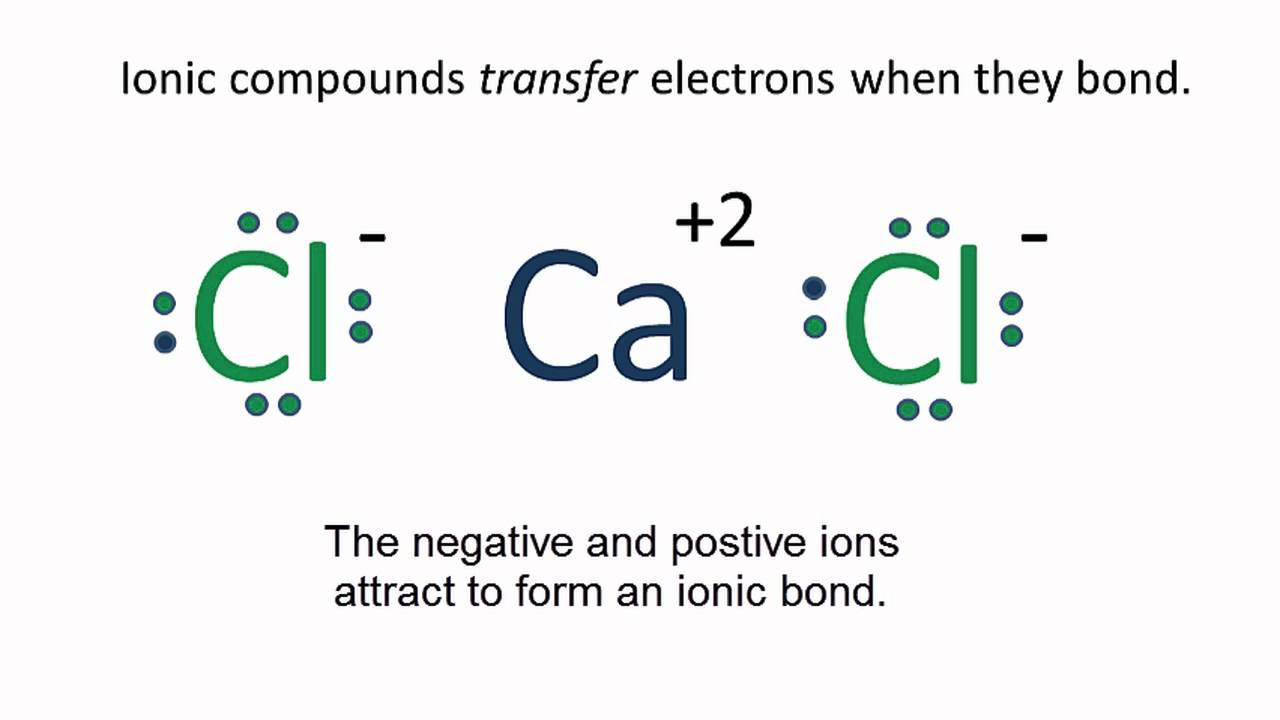
This is because electrons tend to occupy . Chromium(6+) | Cr+6 | CID – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.
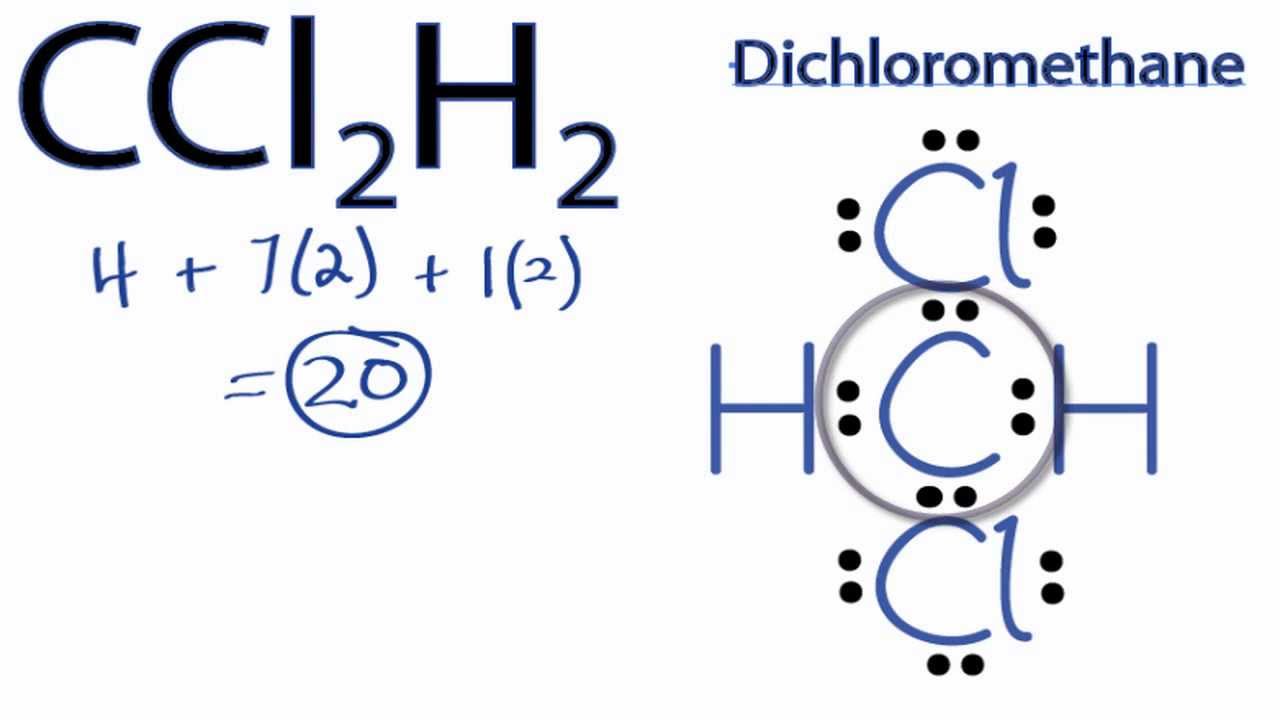
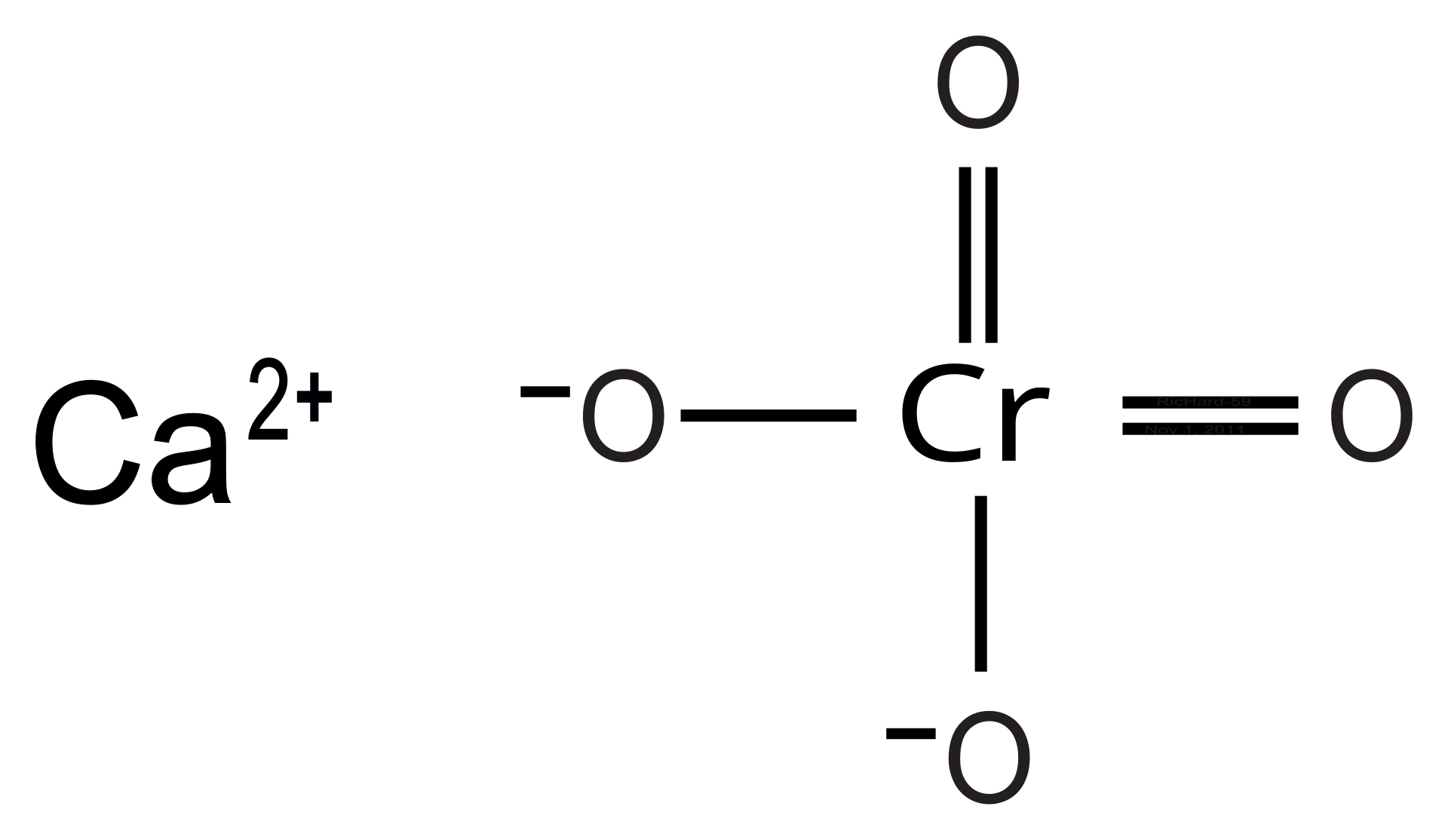
Dot| Lewis structures of chromium trioxide CrO3 |Simple method for writing Lewis structures | Lewis configuration, vita min, draw for, lewis.Chromium is element 24 and has electron structure [Ar]3d5 4s1, with 6 valence electrons. Chlorine is element 17 with electron configuration [Ne]3s2 3p5, with 7 valence electrons.
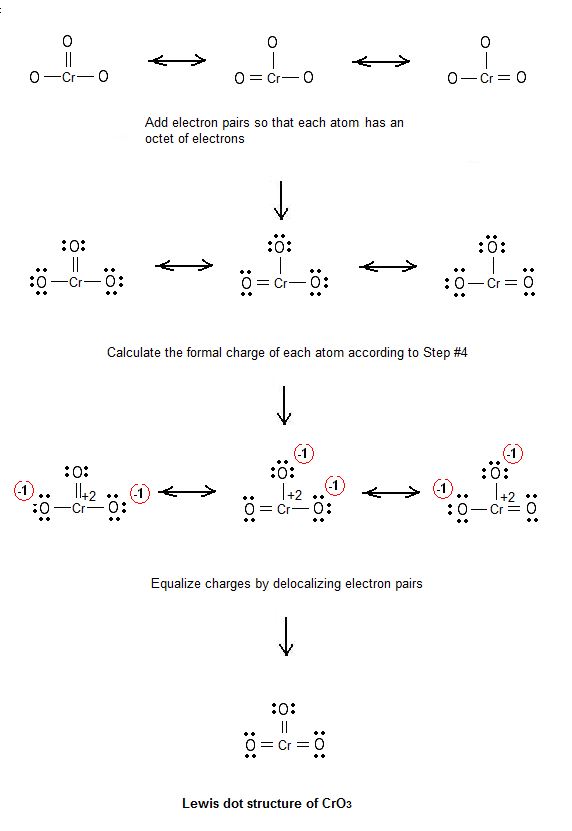
In the +4 oxidation state, Chromium has lost 4 electrons, meaning that Chromium(IV) chloride will have the chemical. Chromium (Cr) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.

Chromium. Because Chromium has 24 protons/electrons on a Bohr Diagram it will have 24 dots. On a Lewis Dot it will have 6 dots because of Chromium’s group number.
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
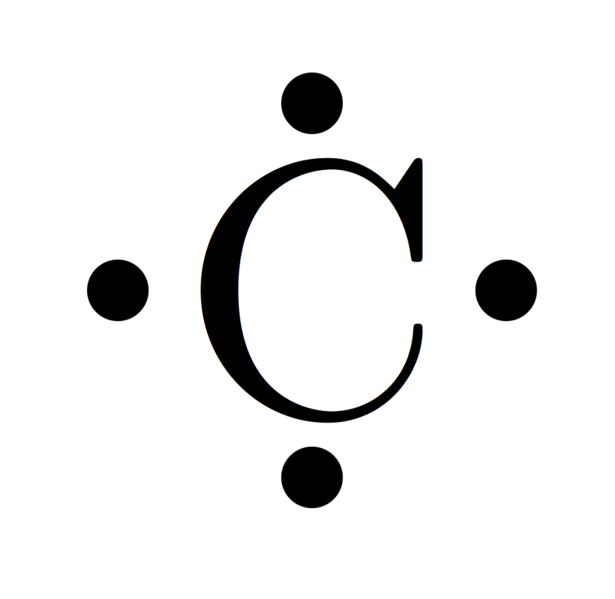
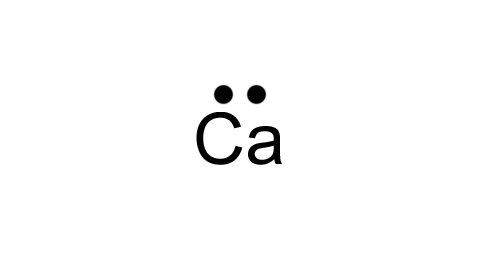
The Lewis dot structure is a diagram to show the bonding between the atoms of a molecule and pairs of electrons that may exist. The Lewis dot structure for chromium is Cr with two dots on top and bottom, and four dots on both sides.Periodic Table of Elements: Chromium – Cr (schematron.org)Show me a picture of the Lewis dot structure for chromium