Viewing Notes: The Lewis structure for SO is requires you to place more than 8 valence electrons on Sulfur (S).
You might think you’ve got the correct Lewis. 70 More Lewis Dot Structures. S does not follow the octet rule.
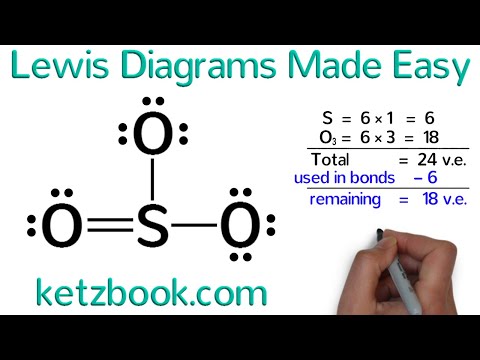
It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also .
Lewis Structures for N2. Step-by-step tutorial for drawing the Lewis Structure for N2. Find the Lewis Structure of the molecule.
(Remember the Lewis Structure rules.) CNS lewis schematron.org 2. Resonance: All elements want an.
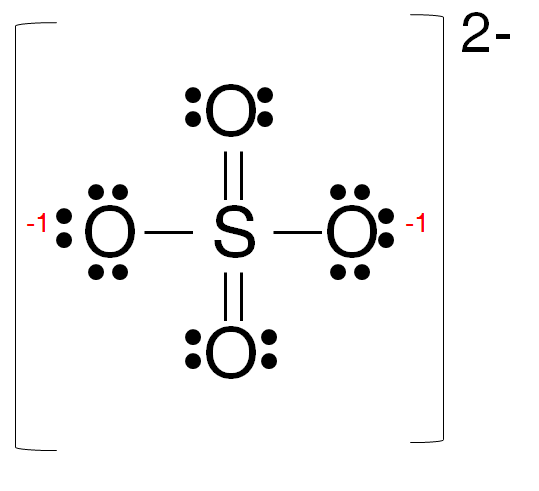
70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons.
Sulfur having valence electrons in the 3rd energy level, will also .The Lewis dot diagram for Platinum is a diagram showing bonds & electrons of the Platinum atom within a molecule. Nobody will be able to draw you a diagram here, as this is a text-only answer board. Use Google Image Search if you want to actually see the Lewis diagram for Pt.

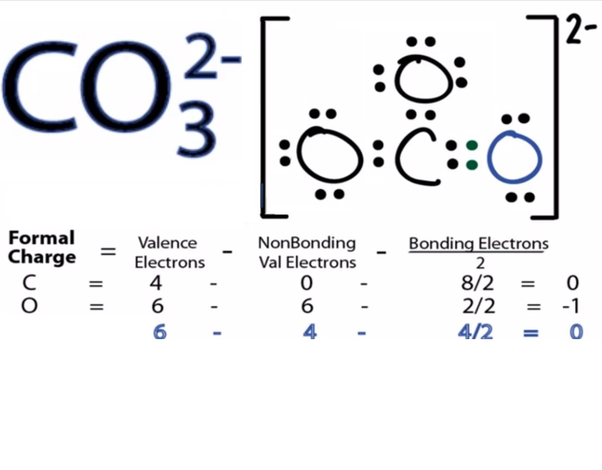
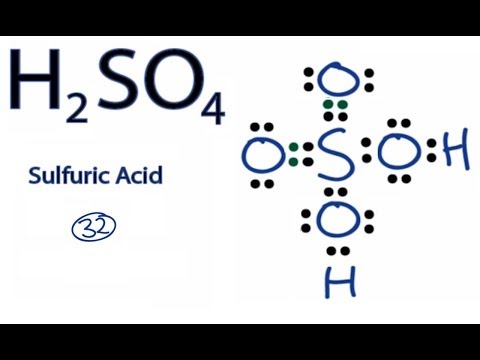
Let’s do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons.
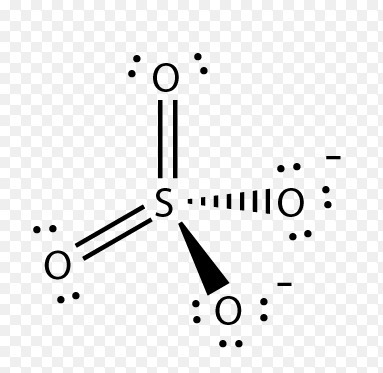
Sulfates (salts with the SO 4 2-) are frequently used in industry and biologically. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo, toothpaste, etc.
MgSO 4 is also known as Epsom Salts. There are 32 valence electrons available for the Lewis structure for SO 4 2. Sep 01, · With lewis dot diagrams you only dot in the outer electron shell (8 dots) SO4 has 8 dots around it each in sets of 2’s like colons (:) and then O’s all around the S next to the dots then finish it of by putting 6 more dots around each O (oxygen)Status: Resolved.

Lewis Dot of the Sulfate Ion. SO 4 2-Back: 70 More Lewis Dot Structures.
S does not follow the octet rule. It will hold more than 8 electrons.
Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons.Lewis Dot of Sulfate SO4 2-Lewis Dot of Sulfate SO4 2-