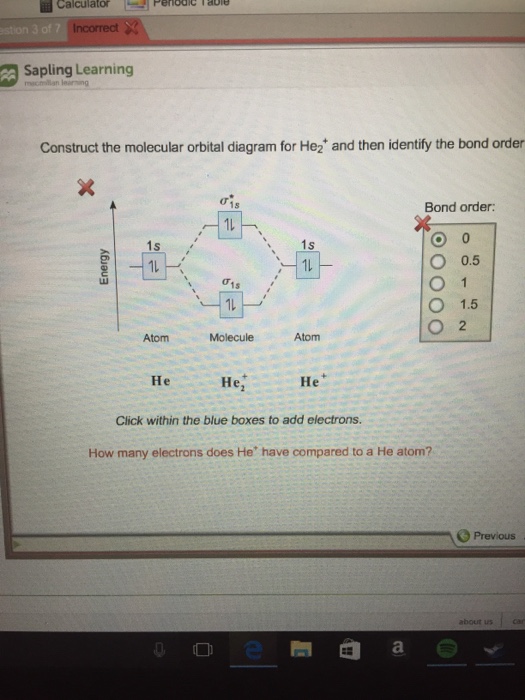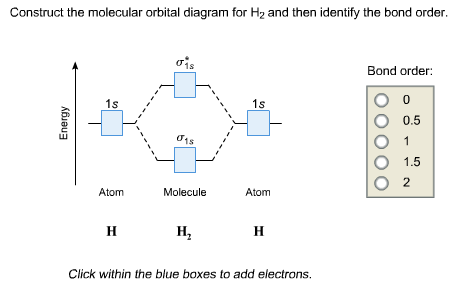
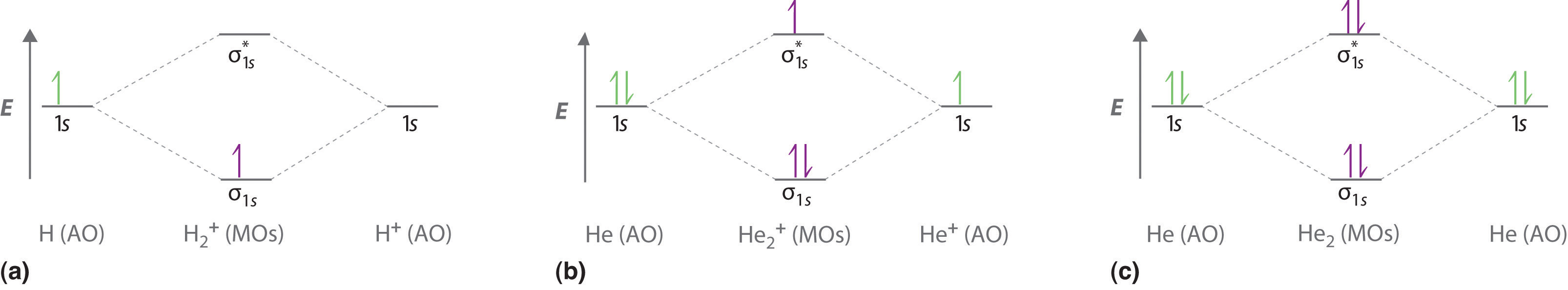
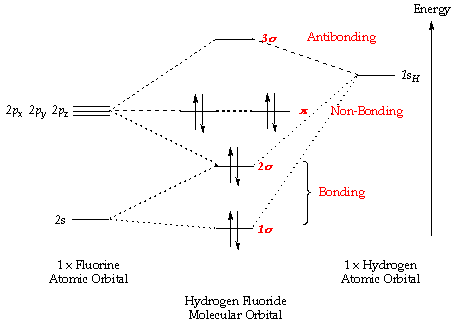
The molecular orbital energy level diagrams for H2, H2.
+, H2. – and O2 H2.
– will be longer. Both have bond order of , but H2.
– is a multi-electron If one nm photon excites two molecules, then half as much energy is will be .. Indicate the lowest energy electron excitation in this ion by identifying the initial and.
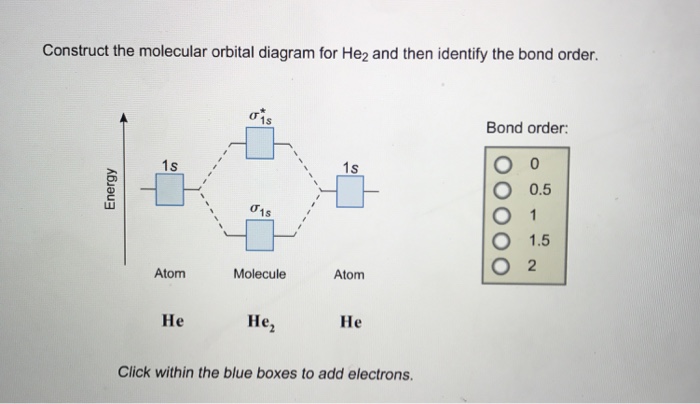
one electron in bonding orbital; so, bond order 1/2. Apr 26, Construct the molecular orbital diagram for h2- and then identify the bond order.
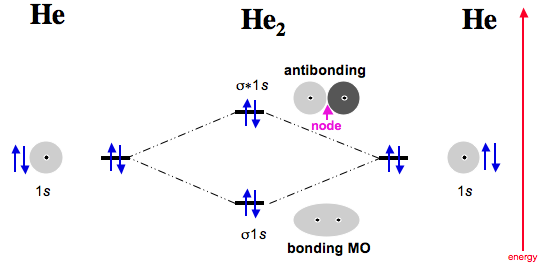
Ask for details; Follow; Report. by Jbugluv 04/26/ Addition of two orbitals can lead to bonding MO and anti-bonding MO. On this page MO for schematron.org Figure1: MO diagram for H2. The filling of Bond order = 1/2 (#e- in bonding MO – #e- in antibonding MO).
For H2, bond. From that diagram, you can then easily fill out what the O2- and O2+ MO So the negative ion has a lower bond order by 1/2 than the neutral molecule.
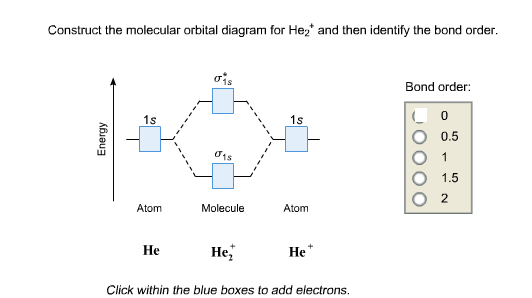
as you can get (apart from H2) and if you don’t understand how to construct it from.Show transcribed image text Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Bond order: Click thin the blue boxes to add electrons.
CHEMICAL FORMULAS AND EQUATIONS. Chemical formulas and equations may contain electron dots, bonds, mathematical operation and comparison signs, chemical arrows, and other notation. The article is composed of three parts.
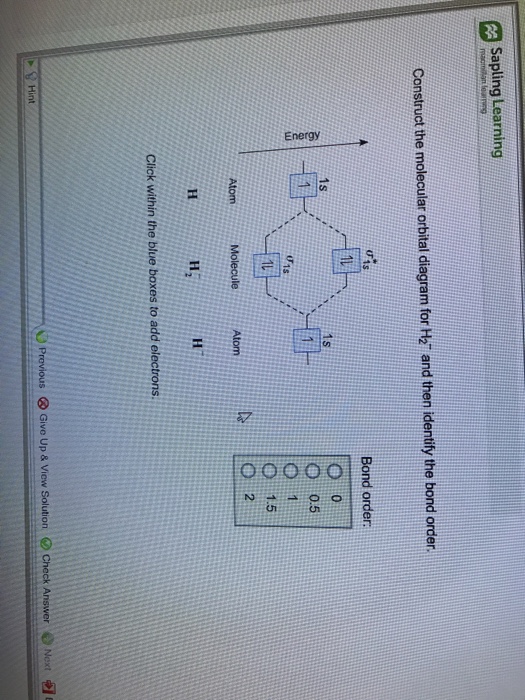
The first section depicts the rationale for space mining and describes the current and future technological state of this field. Nov 02, · For the ion H a) Draw the molecular orbital diagram.
b) Calculate the bond order. c) Would this ion exist?
d) Write the electron configuration of the ion. Since both molecular ions have a bond order of 1/2, they are approximately equally stable.
Problem: Surprisingly, the hybridization of the starred oxygen in the following molecule is sp 2, not sp 3.Construct the molecular orbital diagram for H2– and then identify the bond order? | Yahoo AnswersMolecular Orbital schematron.org?

Help please? | Yahoo Answers