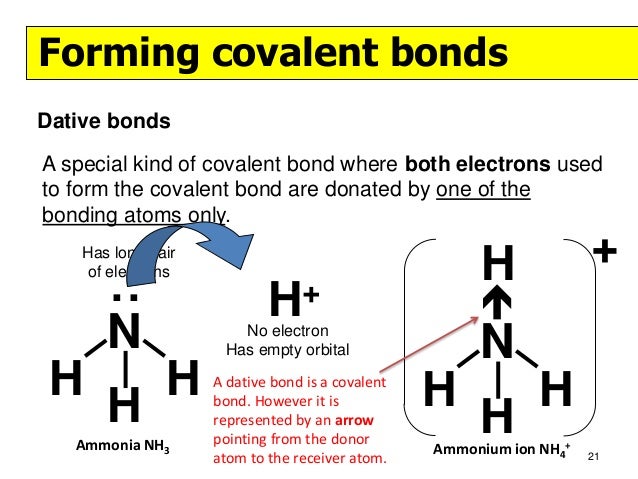
NH4+ is known as an ammonium ion, but NH4 would not make sense as it is not balanced.
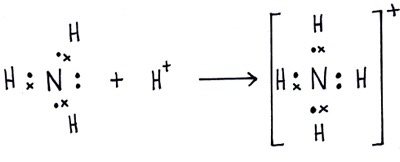
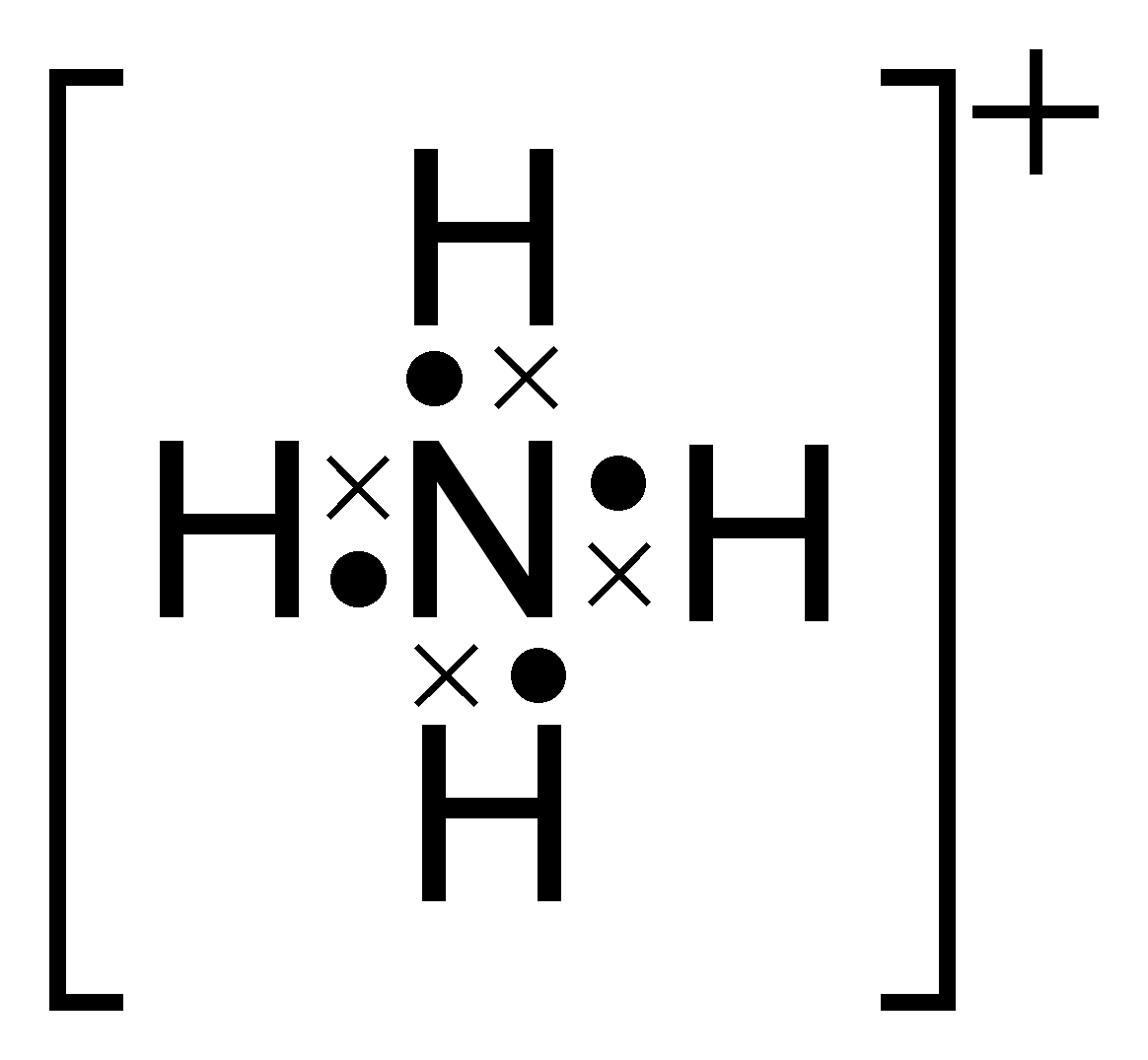
The lewis structure would be the Nitrogen atom in the center, with 4 How can you determine the Lewis dot structure of ammonium phosphate (NH4). Nitrogen has 5 electrons and 4 hydrogens give 4 electrons. As you have a positive charge you must remove one electron, so you have 8.
NH4+ Lewis Structure – How to Draw the Dot Structure for NH4+ (Ammonium Ion) . lewis structure how to draw the dot structure for – 28 images – lewis dot. (i) Calcium hydroxide and Ammonium Chloride (ii) Sodium Nitrite and By drawing an electron dot diagram show the formation of Ammonium ion.
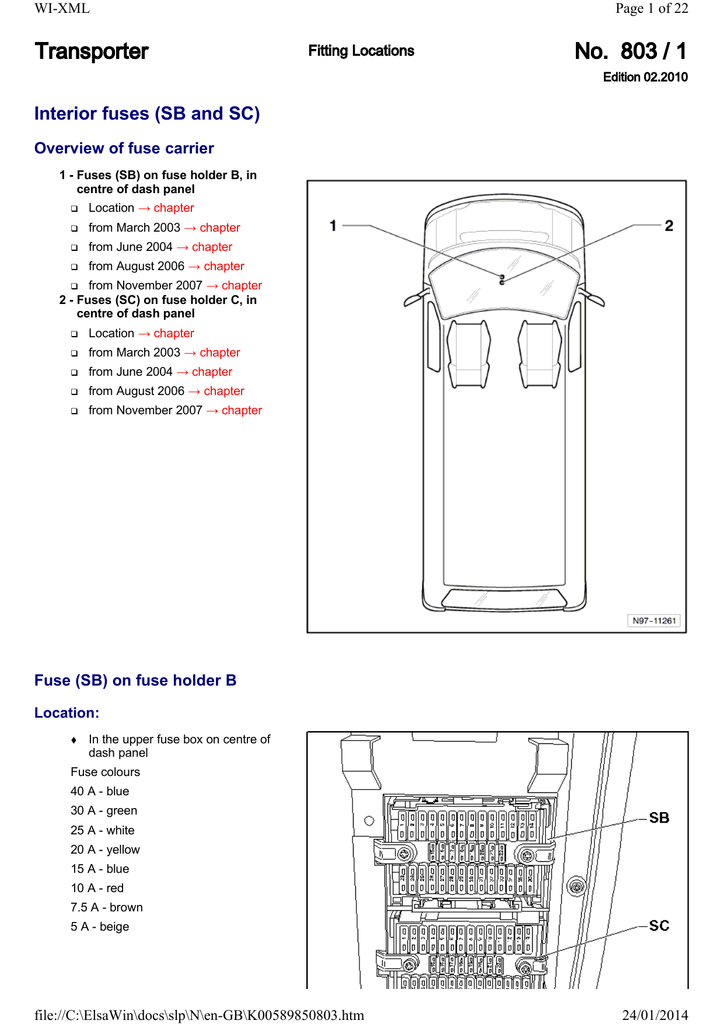
[Atomic No. The Lewis Dot Structure for NH4+ (Ammonium) is shown above.
These kinds In the image above, there are two salts shown which involve the ammonium ion.Dec 02, · Upload failed. Please upload a file larger than x pixels; We are experiencing some problems, please try again. You can only upload files of type PNG, JPG, or schematron.org: Resolved.

Example: Write the Lewis structure for the ammonium ion (NH 4 +). Answer: Hydrogen atoms are always placed on the outside of the molecule, so nitrogen should be the central atom. After counting the valence electrons, we have a total of 9 [5 from nitrogen + 4(1 from each hydrogen)] = 9.
and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram [EDIT] Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen.
Meaning the nitrogen.
a) construct electron-dot diagram for ammonium ion b) determine the electron-pair arrangement and the molecular shape according to VSEPR rules c) for the electron-pair arrangement determines, identify hybridization corresponding to shape d) is it polar or non-polar. Nitrogen has 5 electrons and 4 hydrogens give 4 electrons.
As you have a positive charge you must remove one electron, so you have 8. It simply fills the octet of the nitrogen.Lewis StructureLewis structure – Wikipedia