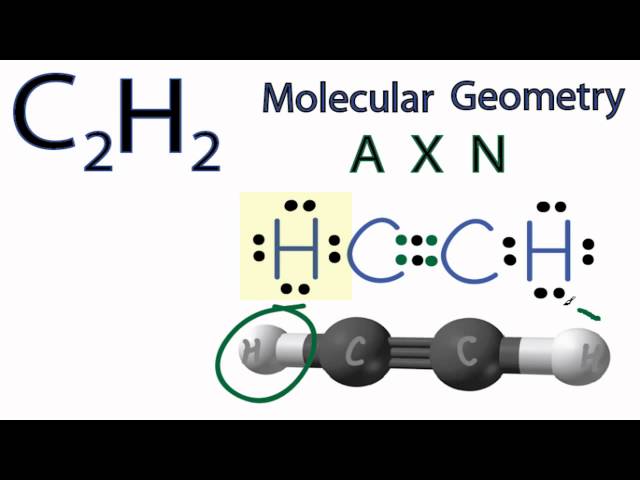
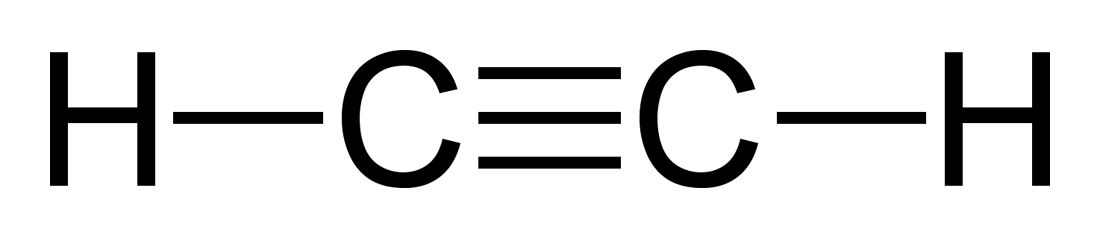
70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule.
The exception, of course, being the. This is a total of 10 valence electrons that have to be included in the Lewis structure. The three lines between the carbon atoms represent 8 valence electrons (2.
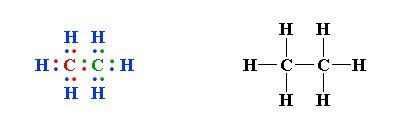
This is a total of 10 valence electrons that have to be included in the Lewis structure. The three lines between the carbon atoms represent 8 valence electrons (2. Drawing the Lewis Structure for C2H2 – Ethyne or Acetylene.
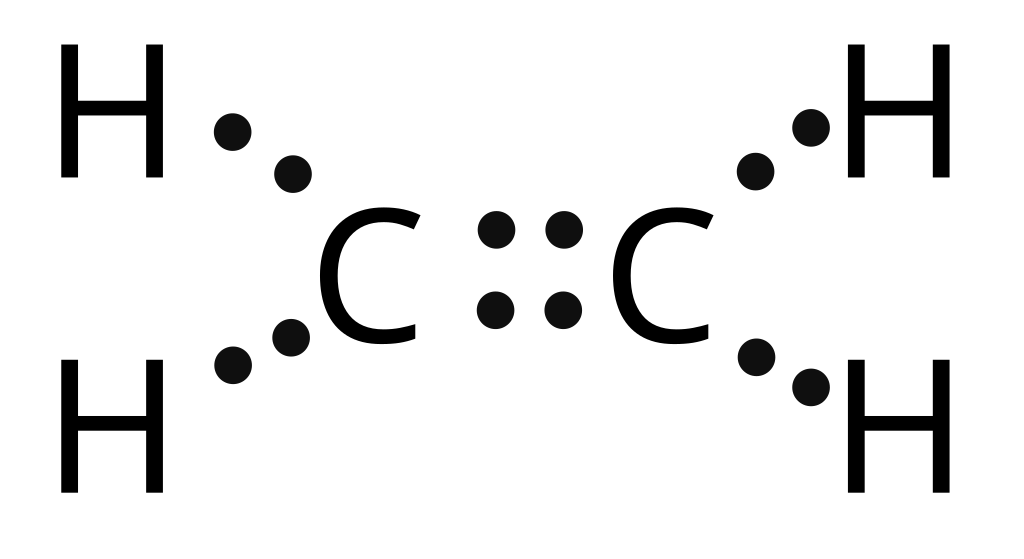
Viewing Notes: With C2H2 you are going to run out of valence electrons and will have to share. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule.
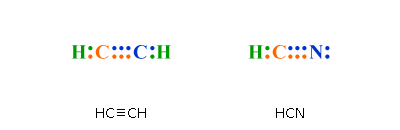
The exception, of course, being the.Nov 01, · This Site Might Help You. RE: i need help on lewis structure for ethyne (C2H2)?
make it simple like “put 2 letter c’s with a line between them. then put 3 h’s around each c with a line between the c and each h” for ethane (C2H6).Status: Resolved. A step-by-step explanation of how to write the Lewis Dot Structure for C2H2 (ethyne or acetylene).
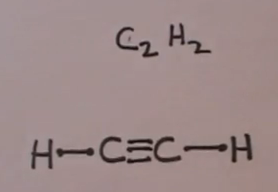
Get more chemistry help at Electron dot structure for Ethyne (C2H2). The difference between the Lewis dot structure and the structuralformula is that the formula only shows the bonds that have formedwhereas the dot structure shows all the valen ce electrons,including lone pairs, in that molecule.

70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule.
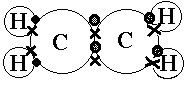
The exception, of course, being the hydrogen’s. They follow the duet rule (2 electrons). Acetylene is an unsaturated hydrocarbon with a triple bond.

Used in . Drawing the Lewis Structure for C 2 H 2 – Ethyne or Acetylene.

Viewing Notes: With C 2 H 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the Carbon atoms. Remember that Hydrogen (H) atoms always go on the outside of a Lewis Structure.Lewis Dot of Ethyne (Acetylene) C2H2i need help on lewis structure for ethyne (C2H2)? | Yahoo Answers