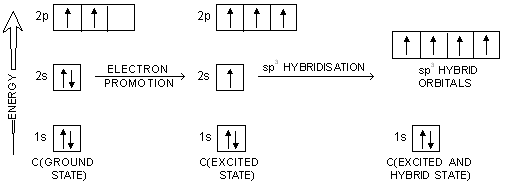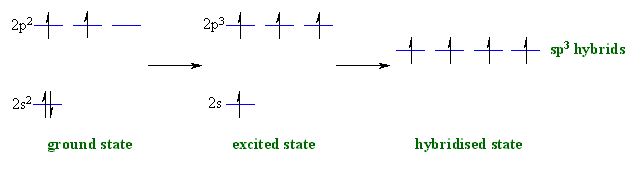
So no, the atom doesn’t have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital.

On this occasion I would love to thank you for seeing this internet site. This moment I will share keyword, I will certainly show to you 10 even.
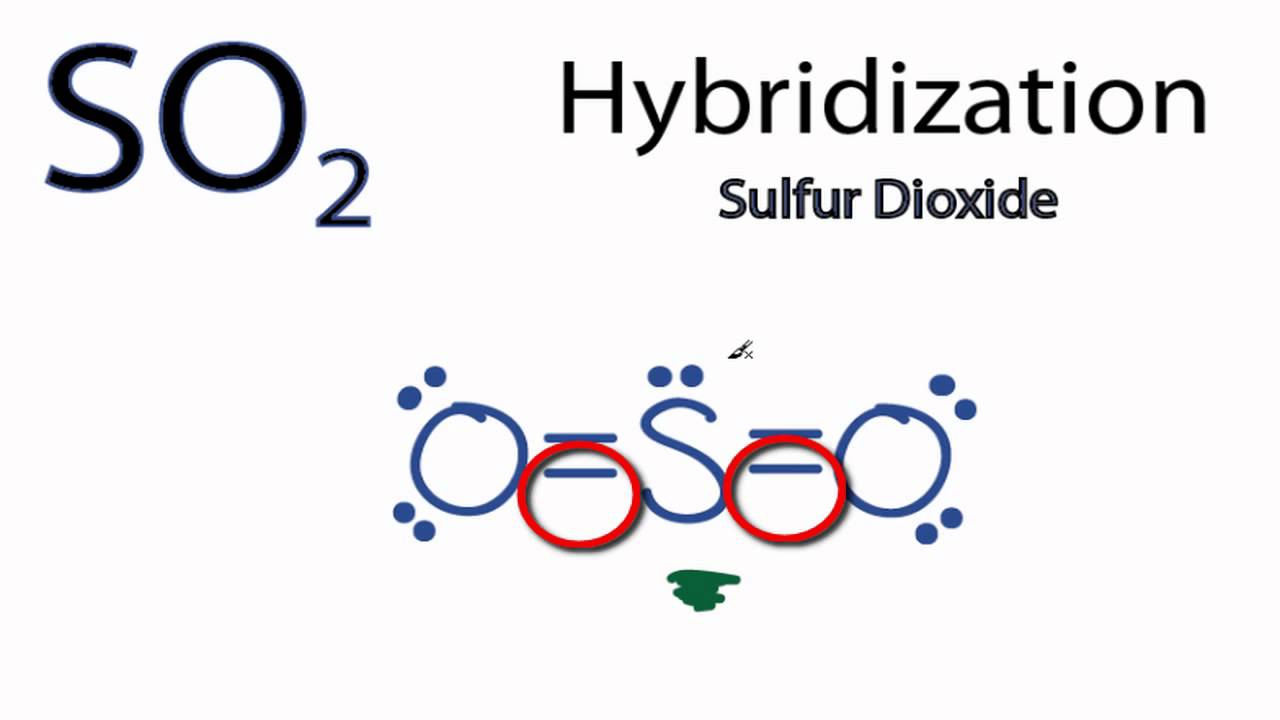
Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in “symmetry” and. Answer to write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization.
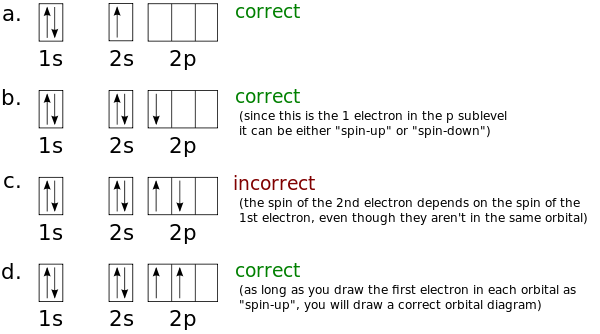
The atomic number of carbon is 6, which is also the number of The orbital diagram shows how the electrons are arranged within each sublevel. rule, each orbital must contain one electron each with the same spin, before.Watch video · Steric number and sp3 hybridization.
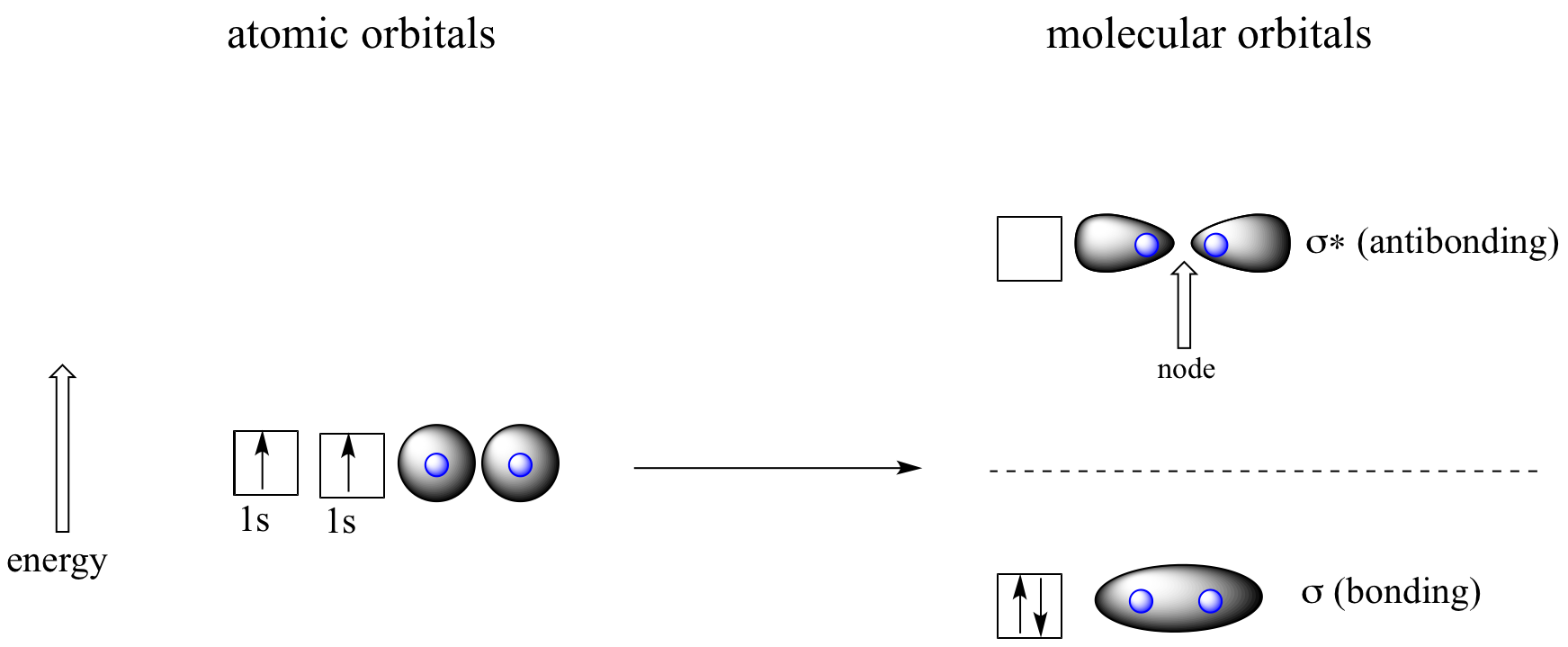
sp² hybridization bond that we formed right here, so here we have an overlap of orbitals, an overlap of an SP three hybrid orbital form carbon, with a un-hybridized S orbital from hydrogen here, and so this is a head-on overlap, so we’re sharing electrons here, in this head-on overlap. of the ethane.
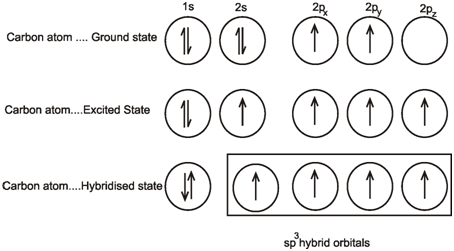
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization. Question: Consider the electron configuration.
Write the orbital diagram of carbon before sp3 hybridization. Orbital Hybridization – sp, sp 2, and sp 3 Carbon.
Hybridization is used to explain molecular structures and describes the various orbital types which are involved in the bonding between atoms. Solution: Consider the electron configuration of a carbon schematron.org the orbital diagram of carbon before sp3 hybridization. Problem.
Consider the electron configuration of a carbon atom. Write the orbital diagram of carbon before sp 3 hybridization.
Next. Practice Problems. Feb 25, · Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks schematron.org: Resolved.Hybridisation – Mixing Up Orbitals with sp, sp2, sp3 | schematron.org – Biochem & Science NotesHybridization of Atomic Orbitals