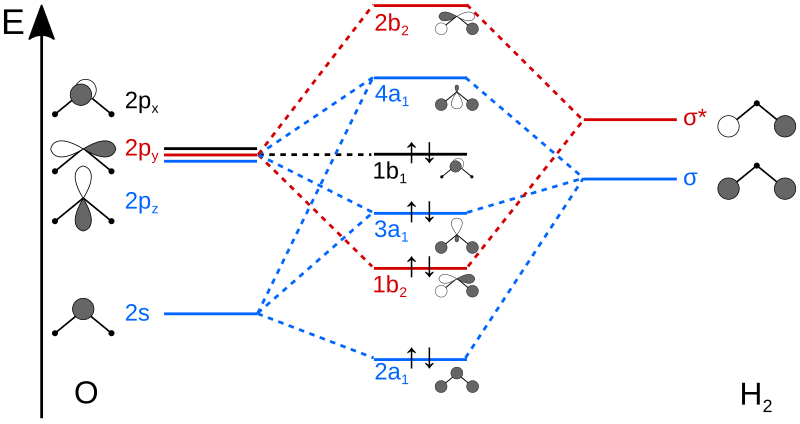
Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−.
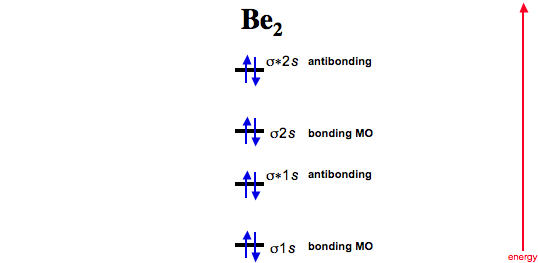
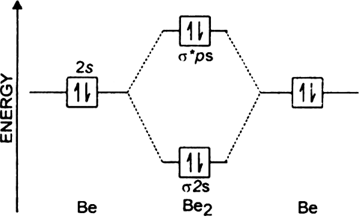
Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−.
Do you expect these molecules to exist in the. Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms.
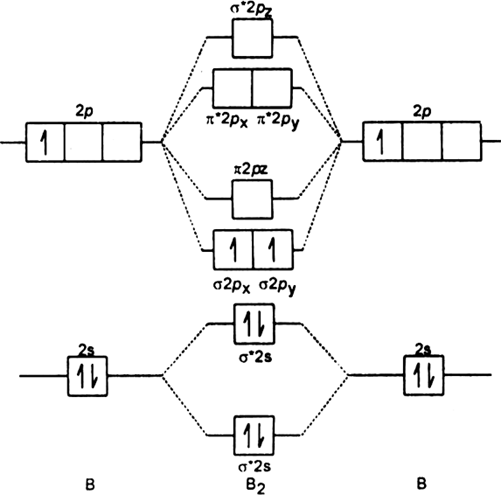
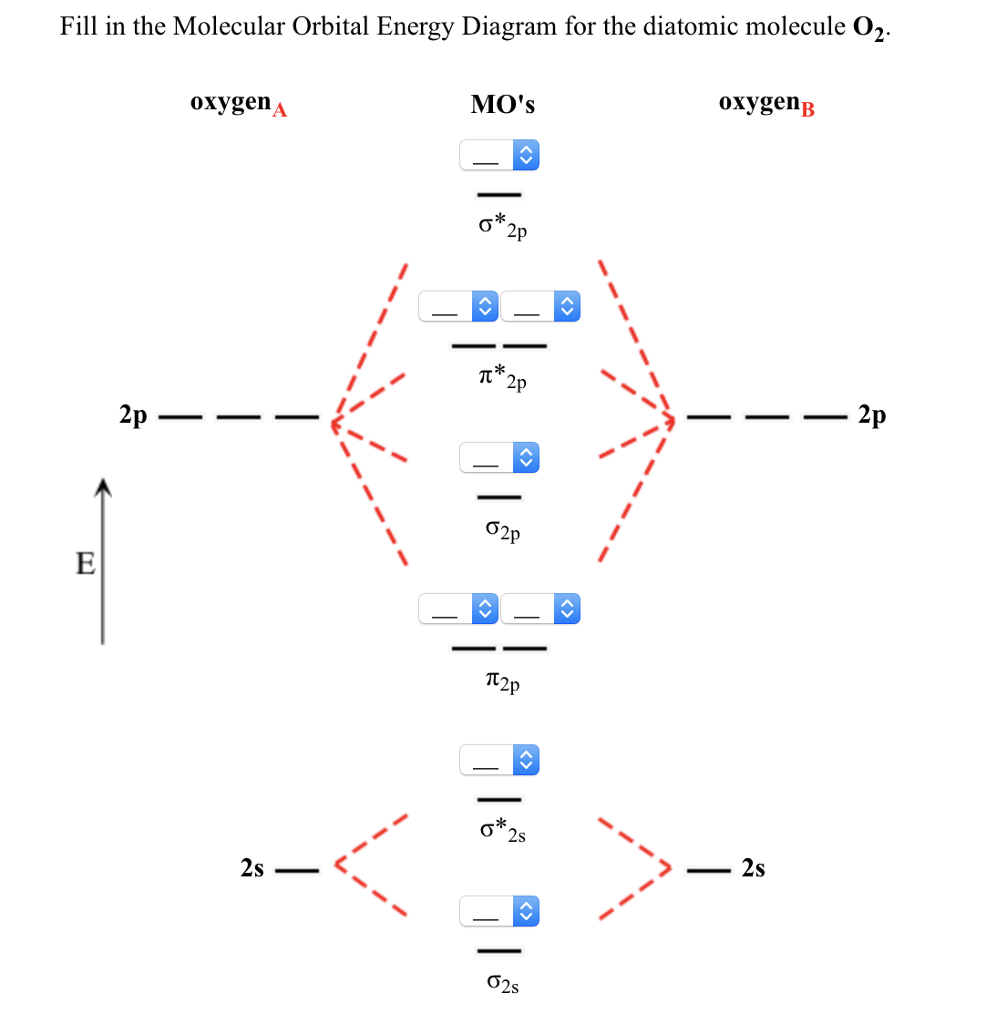
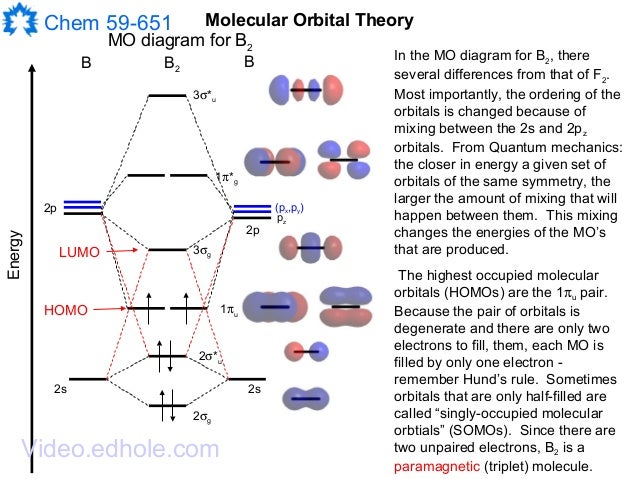
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. Magnetic properties: Since each 2px and 2py MO contains unpaired electron, therefore B2 molecule is paramagnetic. The compound does not exist but that doesn’t mean its MO diagram And From the MOT concept Be2 doesn’t exists as its Border is 0 and in.CAcT Home Molecular orbitals of Li 2, Be 2, to F 2 Skills to develop.
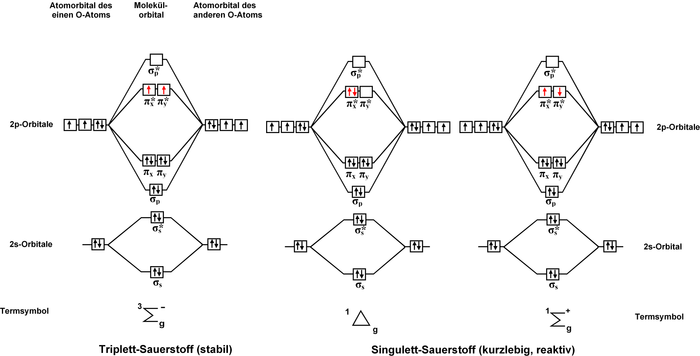
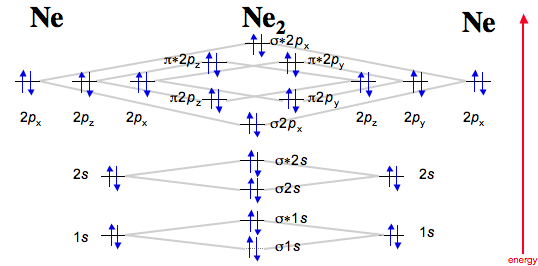
Explain how the energy levels of atomic orbitals vary for H, Li, Be, B, C, N, and O. Draw relative energy levels diagrams for homonuclear diatomic molecules of period 2 elements.
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
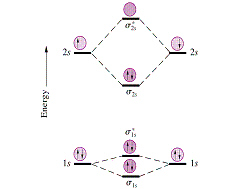
The electrons in each atomic orbital are represented by arrows. In the middle of the diagram, the molecular orbitals of the molecule of interest are written.
Dashed lines connect the parent atomic orbitals with the daughter molecular orbitals. In general, bonding molecular orbitals are lower in energy than either of their parent atomic orbitals.
Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
This example was covered in class to show the rare exception that this single bond is a bond. May 04, · I need help drawing this molecular orbital.
I can draw (Be2)^+ but not this. I know I’ll need a 2p orbital but there’s orbital mixing going on so I have 2 choices:Status: Resolved.Diatomic Species | MO theory | ChemogenesisMolecular orbital diagram – Wikipedia