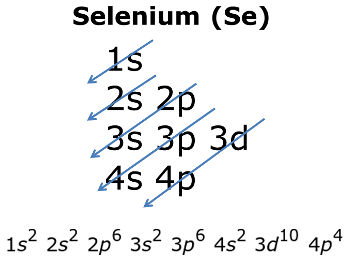
here is the electronic configuration.

Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. First, we consider a possibility of a double bond between the two selenium atoms . The corresponding molecular- orbital diagram for the Se 2 dimer, which is a.
The orbital diagram is the most detailed picture of showing where all the To draw the orbital diagram of selenium we start by determining the number of. The aufbau principle is a method for determining the electronconfiguration of an element.
It shows how the various orbitals mustbe filled in correct sequence to. The electron configuration of selenium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. the periodic table, and the superscript 4 is how many electrons are in that orbital.This shows thatin energy level 1, there are 2 electrons in the s orbital.
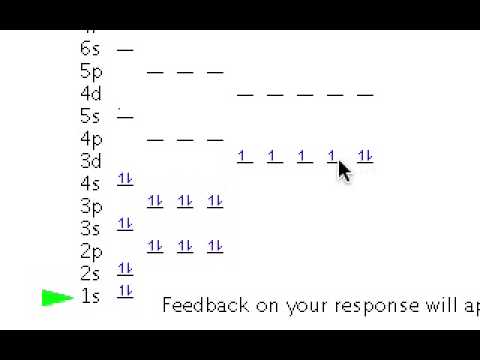
In the2nd energy level, there are 2 electrons in the s orbital and 6electrons in the p orbitals. etc.
The orbital diagram would showessentially the same thing, but would include the spin of eachelectron, by showing up and down arrows. By looking at the electron configuration of selenium, it is possible to determine how many electrons are in each sub-shell.
Molecular orbital diagram
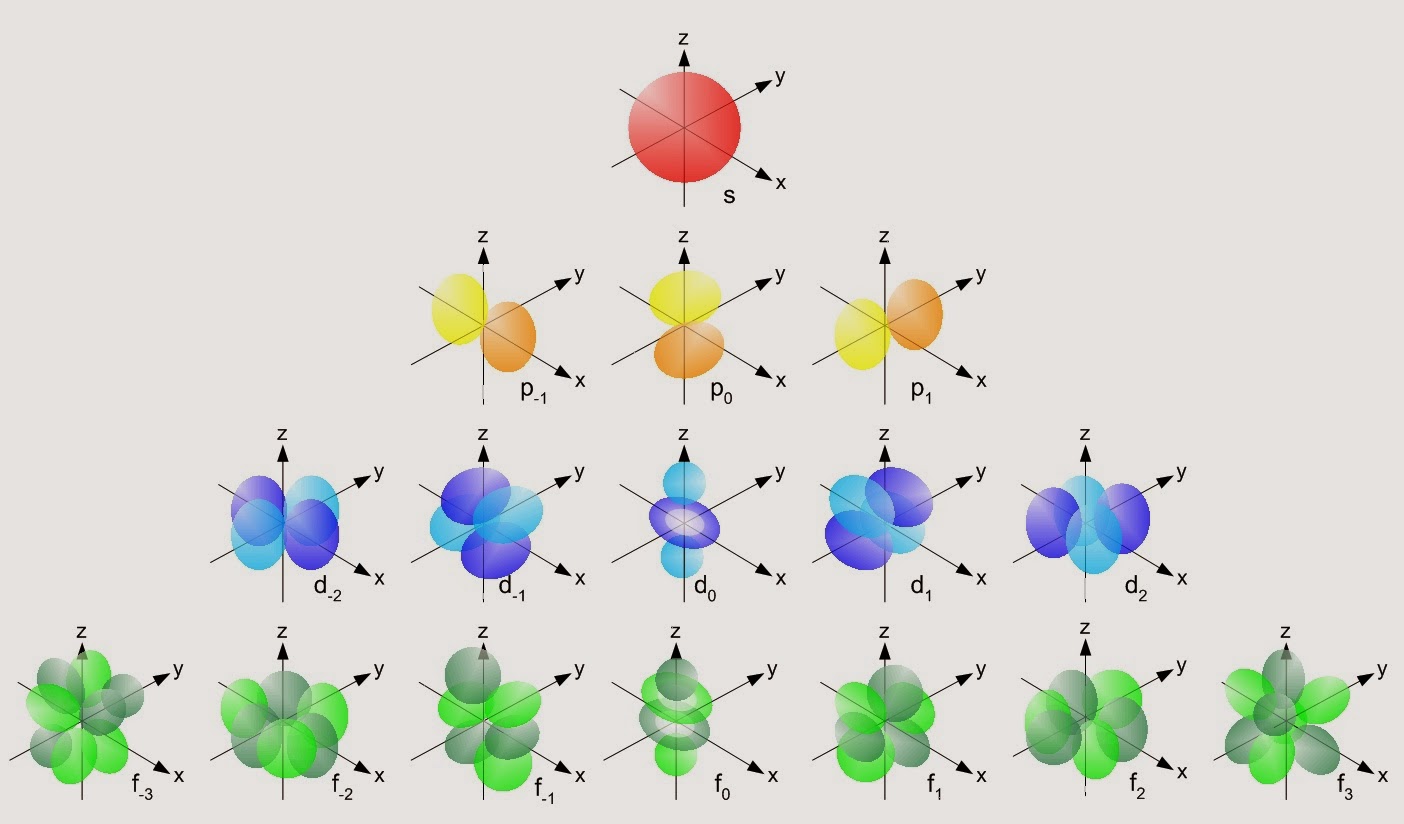
There are five sub-shells, but only four of them are used by naturally occurring elements: s, p, d and f. Each sub-shell accommodates a certain number of electrons.
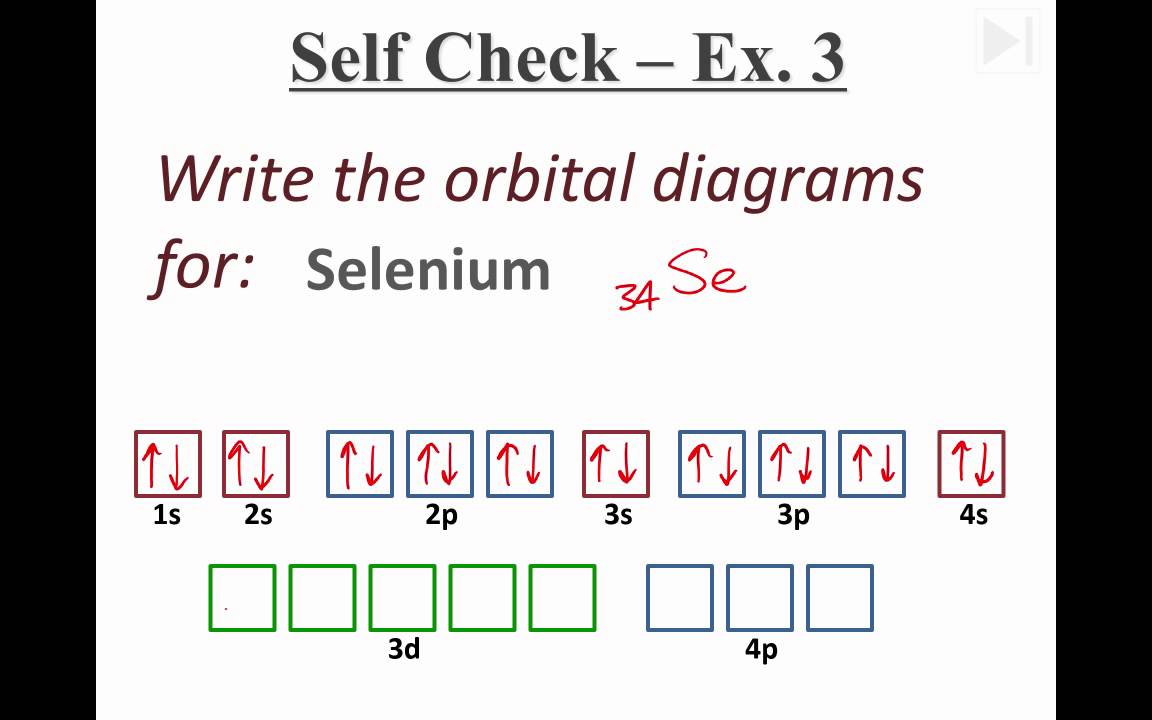
Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Selenium Orbital Diagram – Electron Filling Diagram Iron further File Electron configuration iron as well US together with Electron Configuration For Se together with together with us in addition electron configuration for se along with file electron configuration iron .

Jun 17, · Lewis Structures Made Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules – Duration: ketzbook 43, views.What is the Orbital Diagram of Selenium? | Yahoo AnswersMolecular orbital diagram – Wikipedia