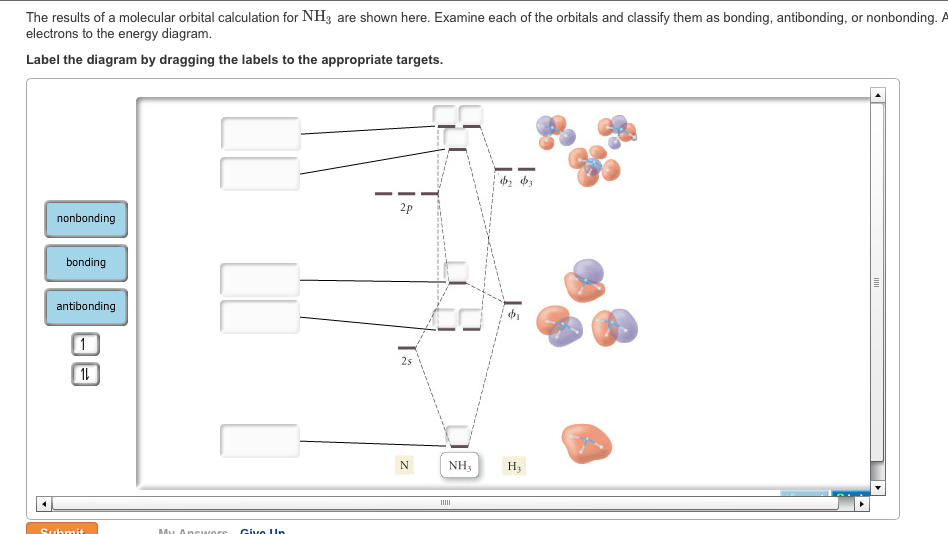
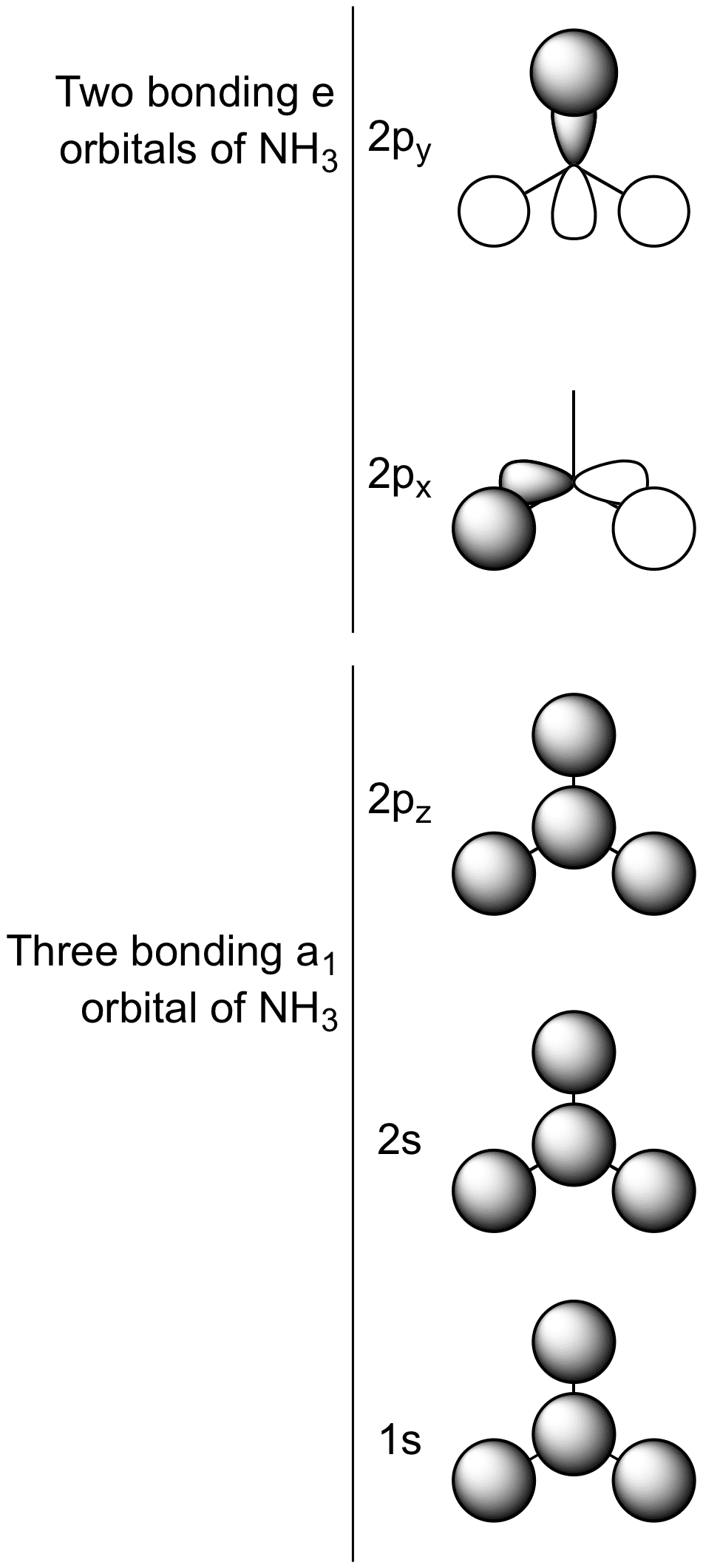
As can be seen from the energy diagram – four of the molecular orbitals occur as Ammonia has two pairs of degenerate orbitals, one bonding and one.
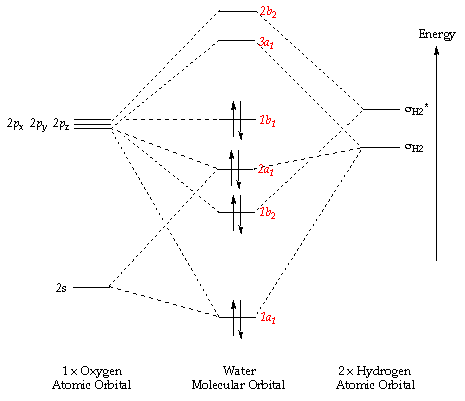
MO diagram of homonuclear diatomic molecules. • Filling the .
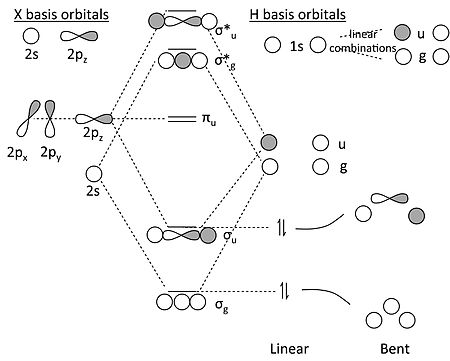
4) MO theory and molecular geometry (Walsh diagrams) . 2) Molecular Orbitals of NH3 (C3v).
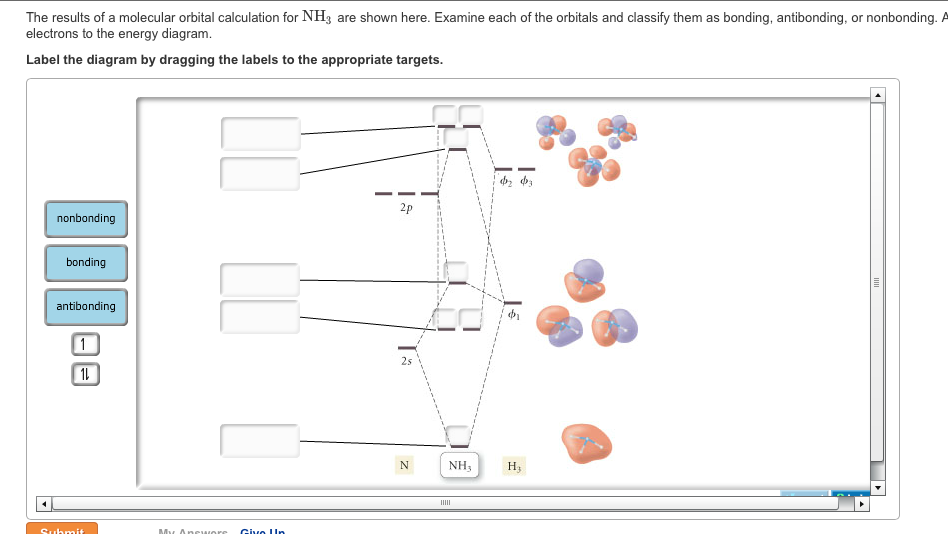
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. I need some help drawing a NH3 molecular orbital. One of the greater problems I have is, that I can’t find MO in LaTeX which are so complex as my diagram.
It is recommended to name the SVG file “Ammonia MO schematron.org” – then the template Vector Introduction to Inorganic Chemistry/Molecular Orbital Theory.MO Diagram for Triangular H 3 A fragment approach to deriving molecular orbitals Inorganic Chemistry. This lone pair orbital also involves bonding of N 2p z with the bonding MO of the stretched H 3 molecule This MO is responsible for the Lewis base character of the ammonia molecule Inorganic Chemistry.
Molecular Orbital Theory – Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – .
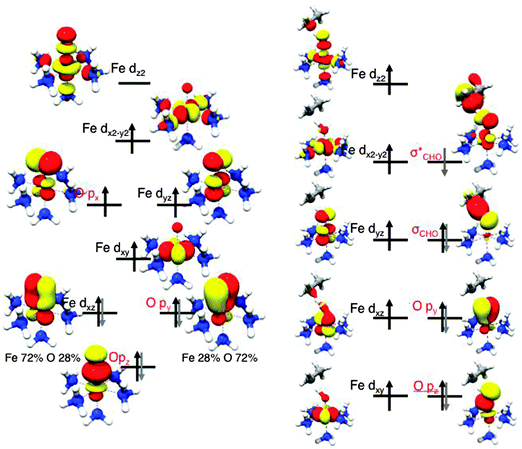
Each N–H σ-bonding orbital, containing 2 electrons, is formed from a N sp3 hybrid orbital and a H 1s orbital. The remaining lone pair of electrons occupies the fourth tetrahedral position producing a pyramidal structure.
Now, here is where it gets interesting. Nitrogen’s energy levels look like this Looking at this energy diagram, one could see that each of the three p-orbitals is available for bonding, so why would the atom need to be hybridized? What is the hybridization of #NH_3#?
Chemistry Molecular Orbital Theory Orbital Hybridization. 1 Answer Stefan.
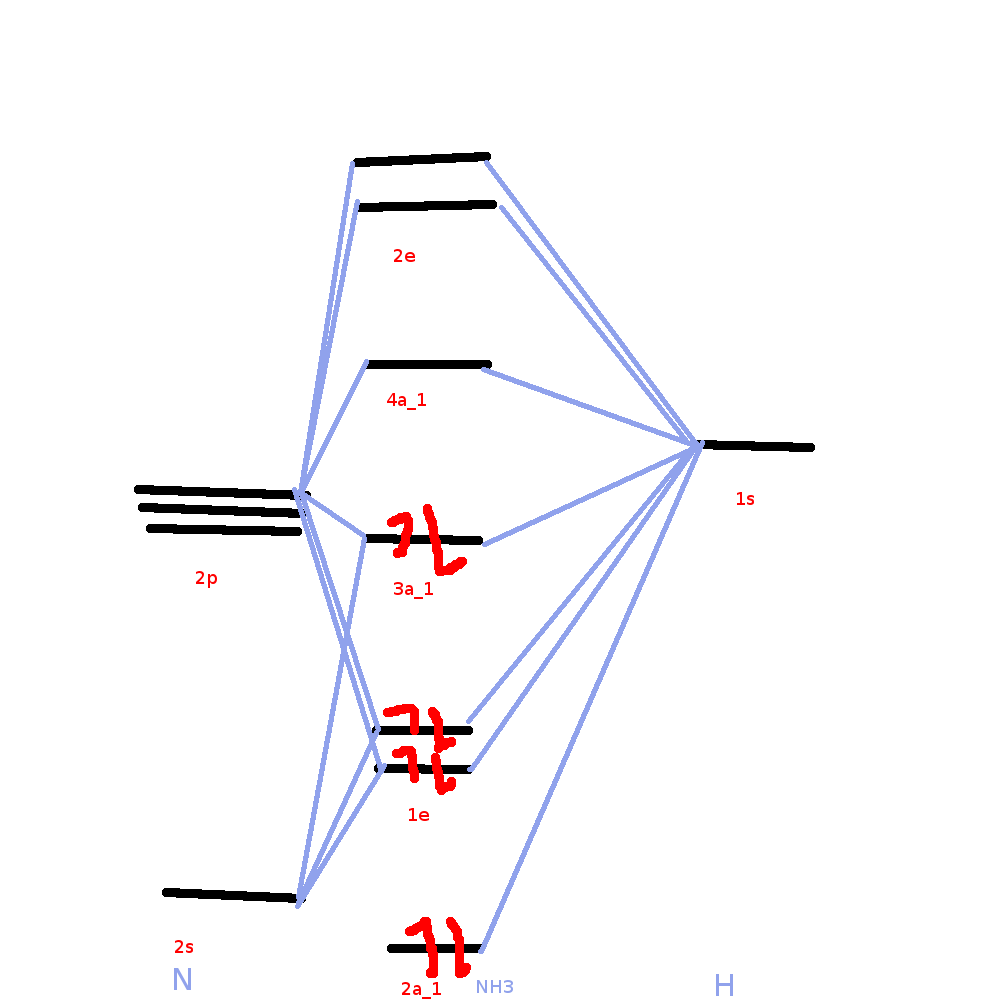
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals.Introduction to Molecular Orbital TheoryWhat is the hybridization of NH_3?
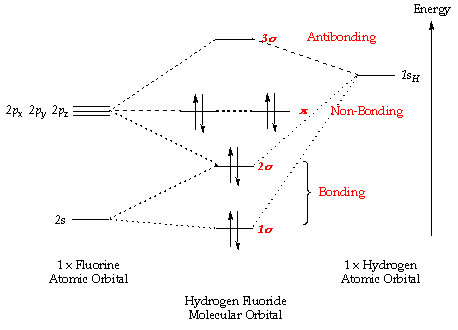
| Socratic