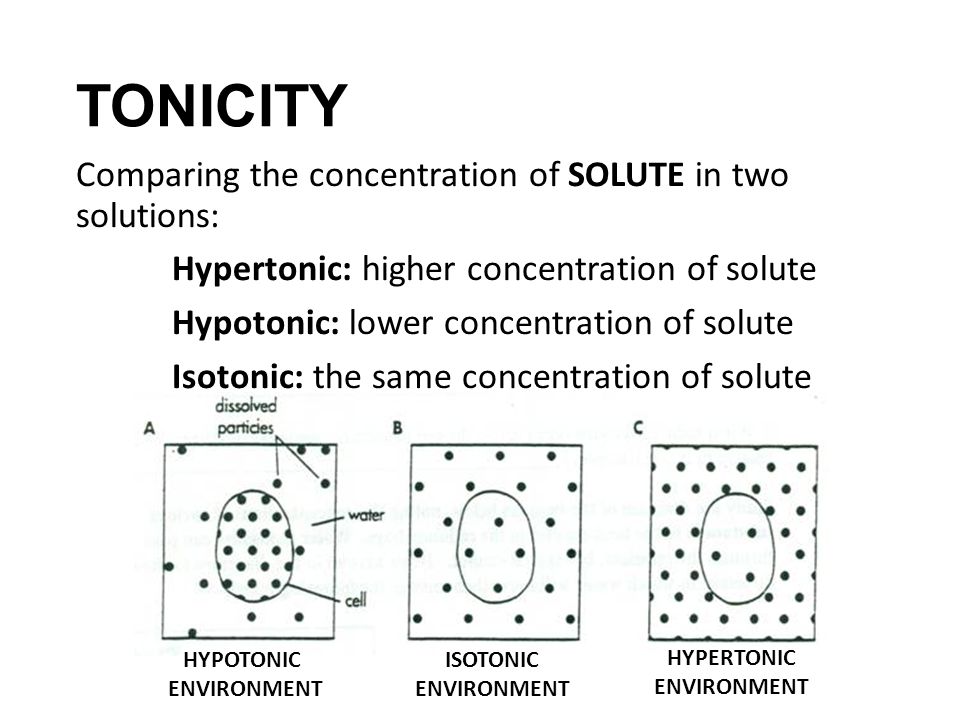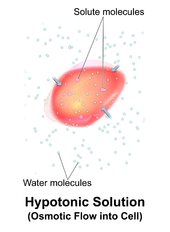
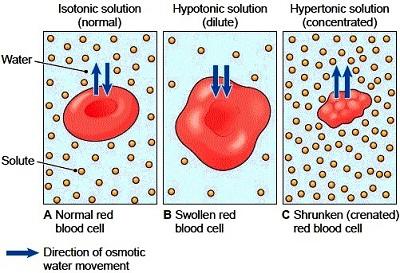
Hypotonic, isotonic and hypertonic solutions (tonicity). Seeing the effect of various types of solution on the direction of osmosis. differences in concentration influence passive membrane transport. Diffusion, Osmosis, and Tonicity.

Simple diffusion. Particles in solution are generally free to .
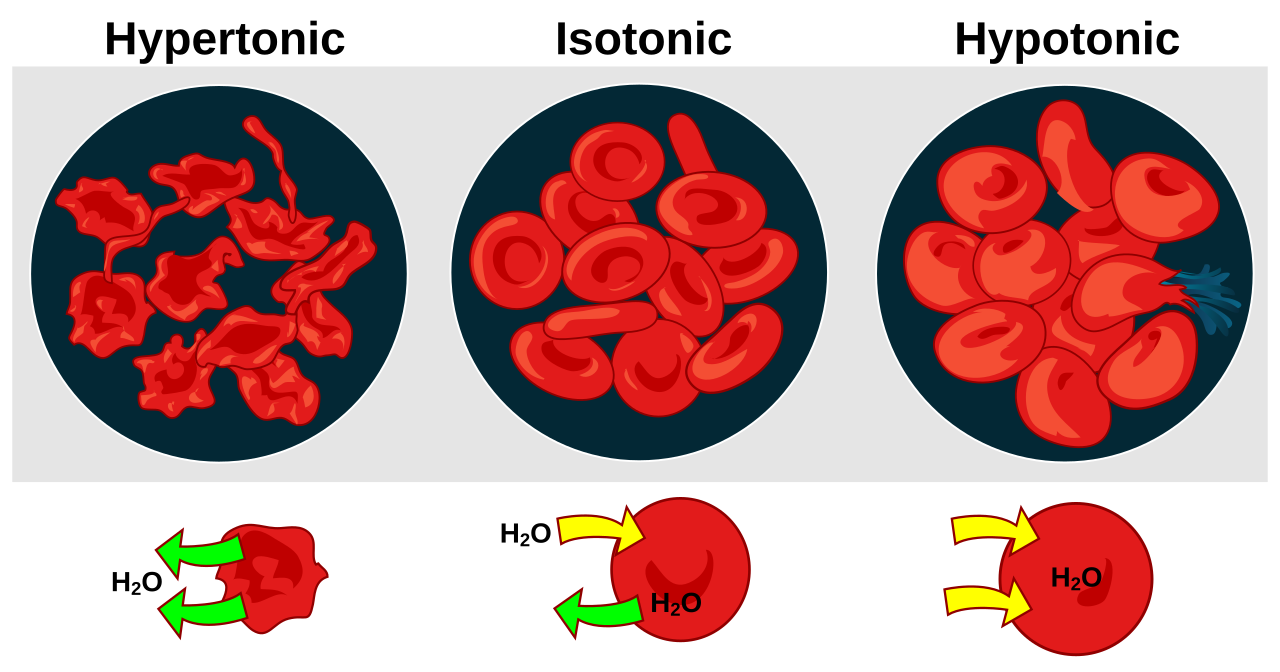
Hypertonic, isotonic, and hypotonic solutions and their effect on cells. Formally, osmosis is the net movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Three terms—hypotonic, isotonic, and.
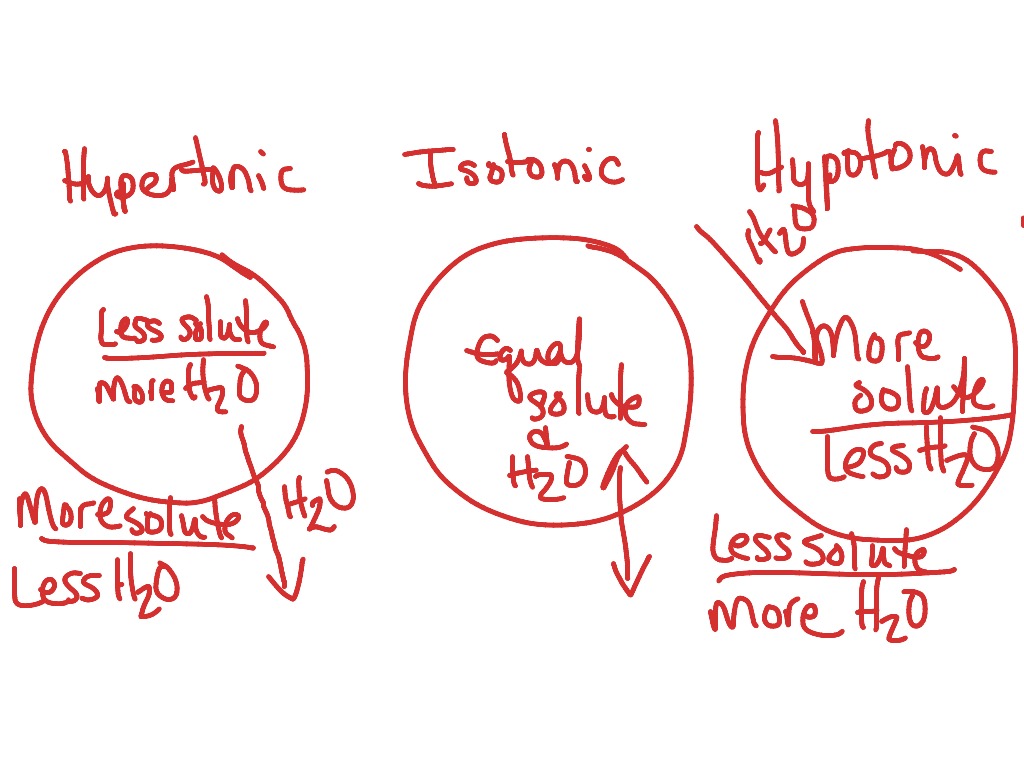
Posts about tonicity written by nabeeluddin1. of the cell is greater than the concentration of water molecules outside the cell, as shown in the diagram below .
Posts about tonicity written by nabeeluddin1. of the cell is greater than the concentration of water molecules outside the cell, as shown in the diagram below .Worksheet – Osmosis & Tonicity READ ME!
In each diagram below, a “cell” with a semipermeable membrane has been placed in a beaker containing substances that are dissolved in water. The membrane is permeable to water & iodine. It is not permeable to glucose, sodium (Na+), or starch. Osmosis and tonicity.
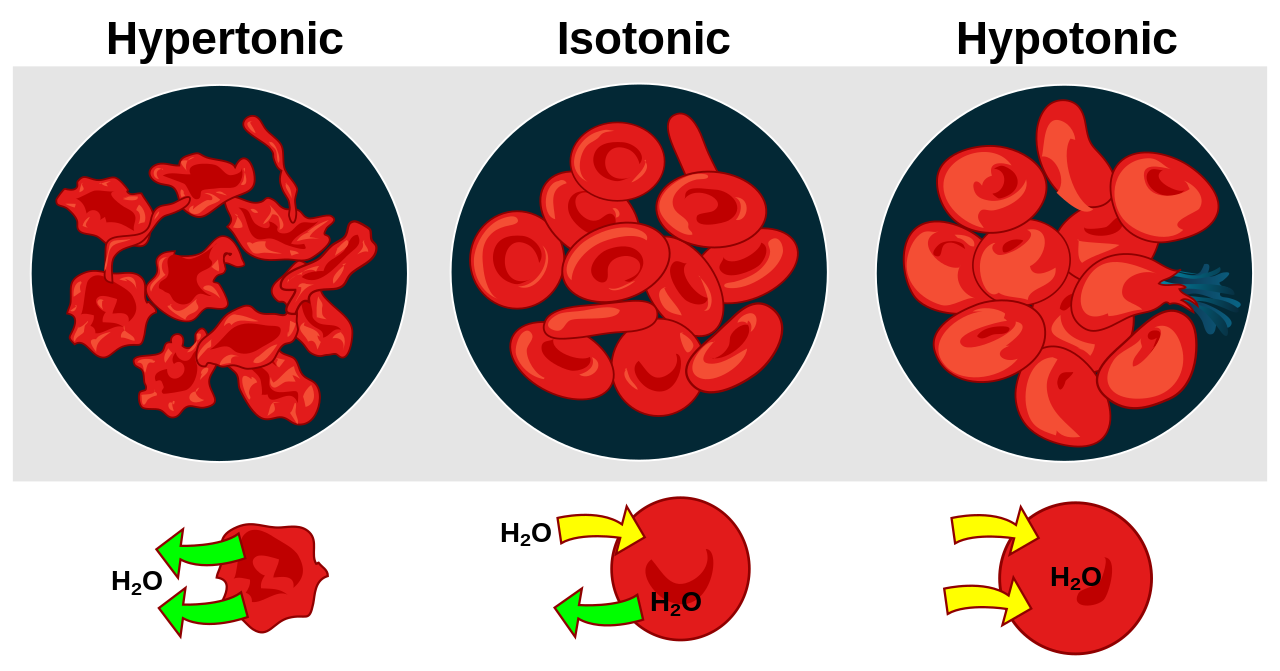
Hypertonic, isotonic, and hypotonic solutions and their effect on cells. If you’re seeing this message, it means we’re having trouble loading external resources on our website. If you’re behind a web filter, please make sure that the domains *schematron.org and *schematron.org are unblocked.
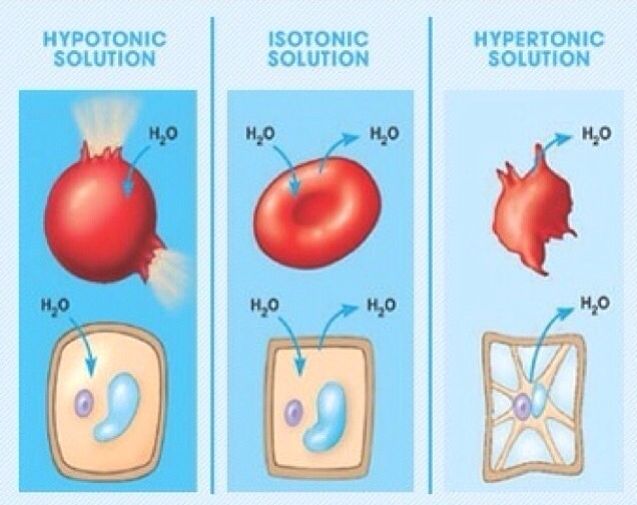
Once again, tonicity is a measure of the osmotic pressure gradient of two solutions separated by a semipermeable membrane. Osmotic pressure gradient is the amount of force needed to keep water. Tonicity diagram practice manager.
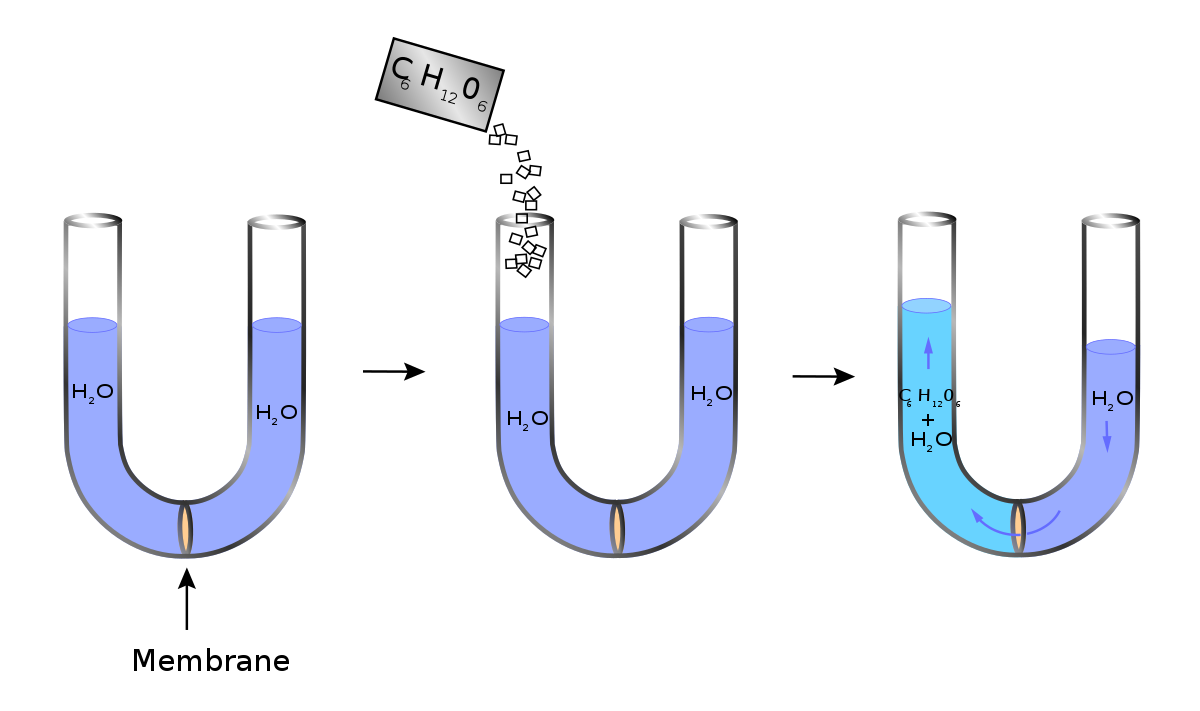
From Royal Adventurers. Jump to: navigation, search. clinical oncology fellowship The purpose of the Fellowship Program is to nursing staff pharmacists social workers and the attending physician to The Penn Oncology Institute .
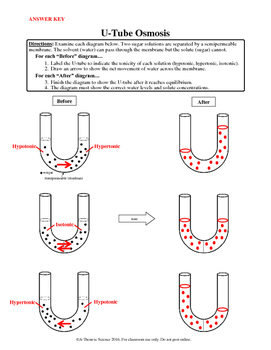
Tonicity is a measure of the effective osmotic pressure gradient, as defined by the water potential of two solutions separated by a semipermeable membrane. In other words, tonicity is the relative concentration of solutes dissolved in solution which determine the direction and extent of diffusion.Tonicity – WikipediaDiffusion, Osmosis & Tonicity: How Osmotic Pressure Impacts Biological Cells