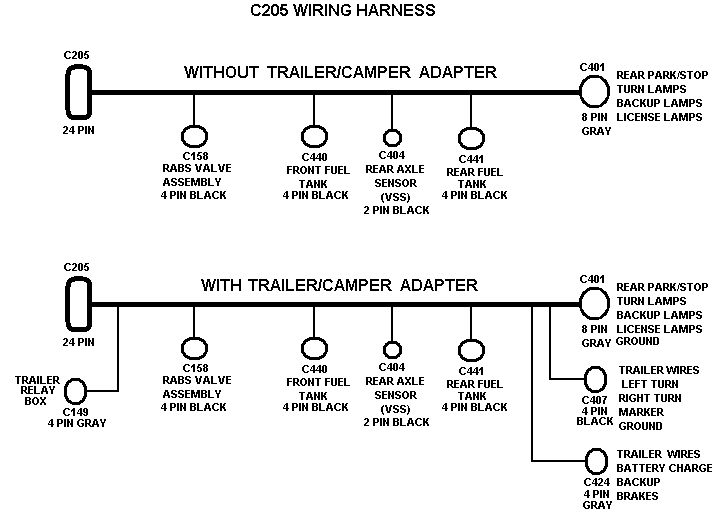
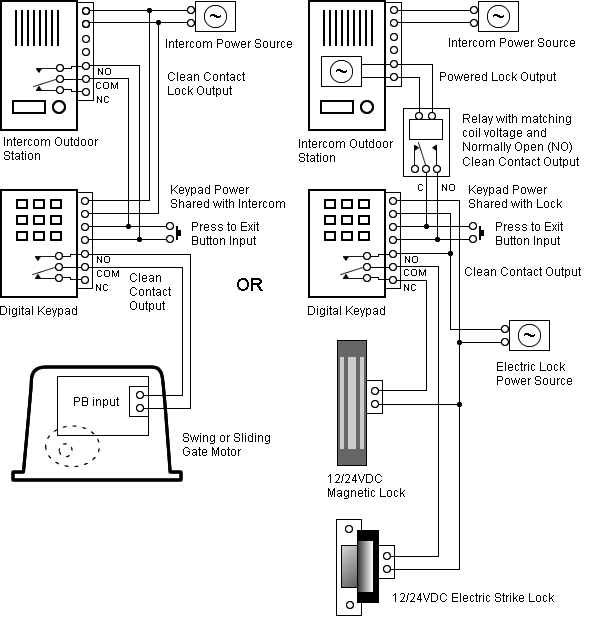
The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two phosphorus; argon.
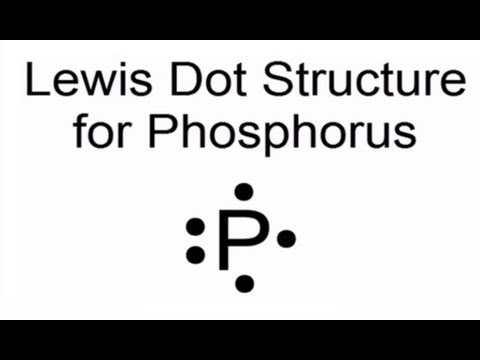
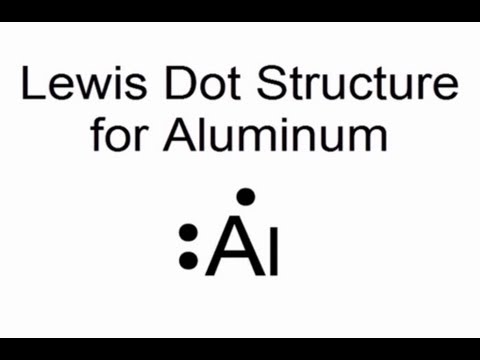
Answer. For atoms with. Phosphorus has 5 valence electrons.
Each electron how to draw it https:// schematron.org Answer: A neutral Phosphorus Atom has five valence electrons. These are Electron Configuration of Phosphorus with a Lewis Diagram on the side as well.
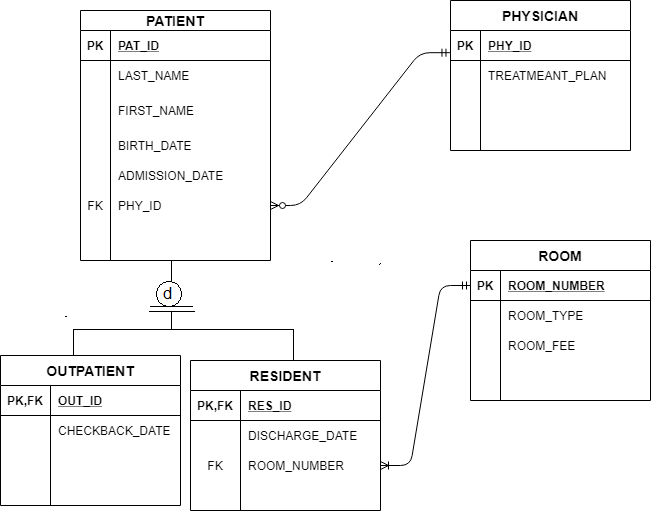
Comprehensive information for the element Phosphorus – P is provided by this page including scores of Atomic Structure of Phosphorus Electron Dot Model .A Lewis electron dot diagram A representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
There are 3xx7+5=26 valence electrons to distribute, i.e. 13 ” electron pairs”.
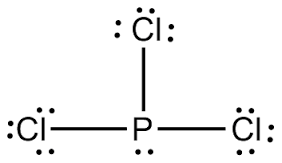
Around EACH bound Cl atom there are 3 lone pairs; there are 3xxP-Cl bonds; the thirteenth lone pair resides on phosphorus::P(-Cl)_3. Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since one of these electron pairs is a stereochemically active non-bonding pair, the.
Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom. For example, the Lewis diagrams for hydrogen, helium, and carbon are.
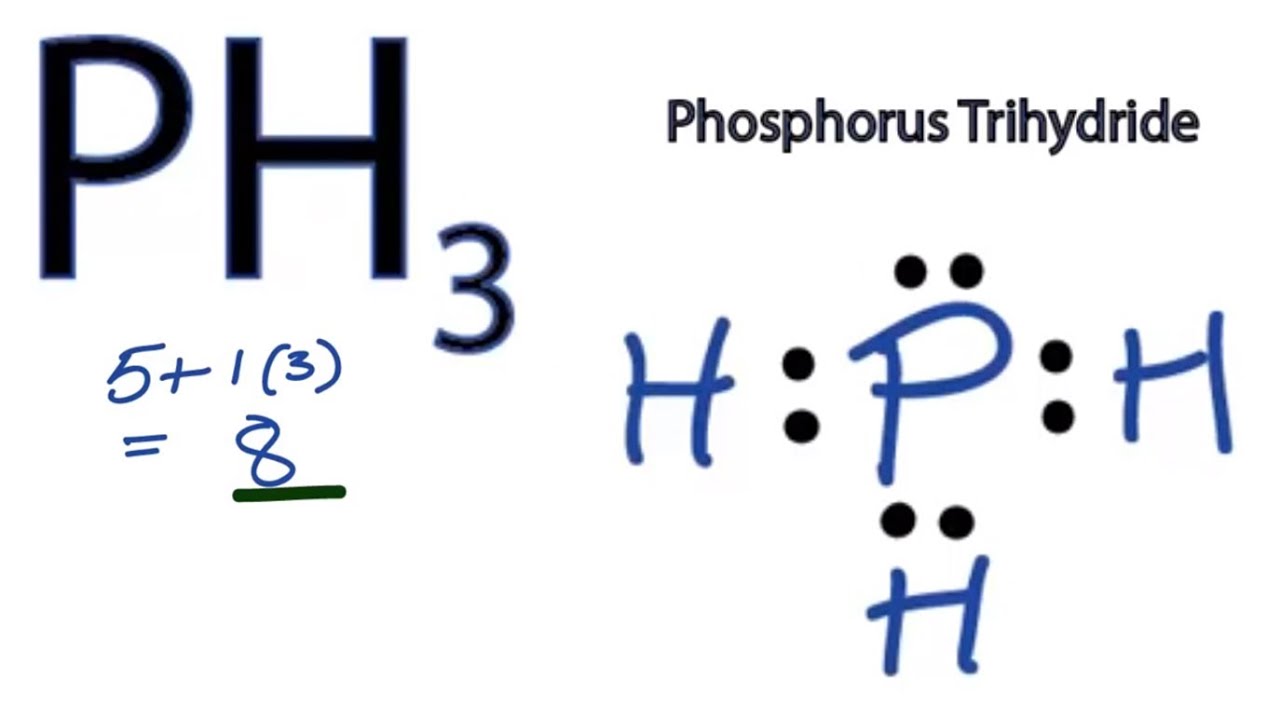
Example: Draw the Lewis structure for phosphorus pentafluoride, PF 5. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
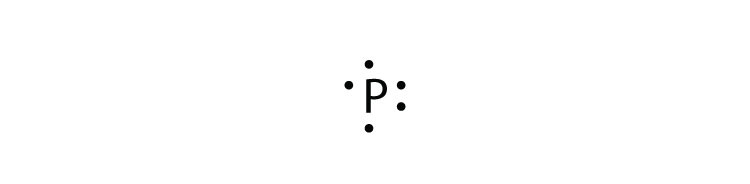
The number of dots equals the number of valence electrons in the atom. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. Lewis Symbols.
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its.Periodic Table of Elements: Phosphorus – P (schematron.org)Lewis dot diagram for phosphorus