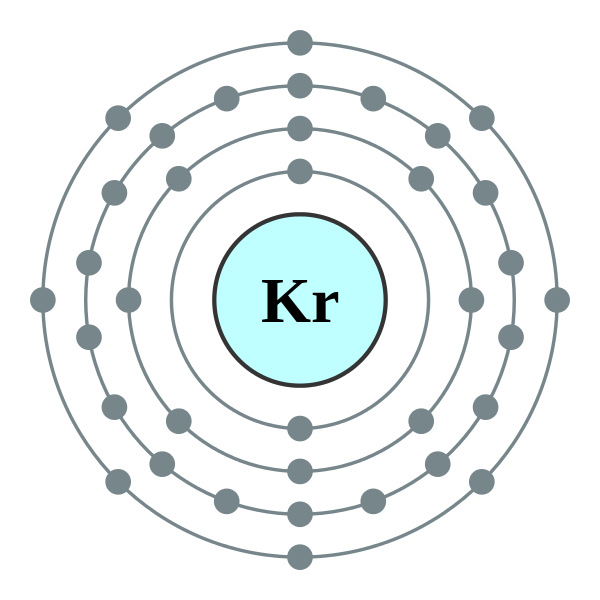
Krypton has a full outer shell of eight electrons, so it would beeight dots, either around the outer circle if you’re doing a fullcircle diagram, or just two dots above, . Since it is in Group 8A it will have 8 valence electrons.
When you draw the Lewis structure for Krypton you’ll put eight “dots” or valance. To answer your question, you would draw the resonance structure of each Lewis Dot Structure and then apply Formal Charge calculation to.
To answer your question, you would draw the resonance structure of each Lewis Dot Structure and then apply Formal Charge calculation to. Comprehensive information for the element Krypton – Kr is provided by this page including scores of Atomic Structure of Krypton Electron Dot Model.The orbital diagram has five boxes with two arrows in the first three and single arrows in the last two.
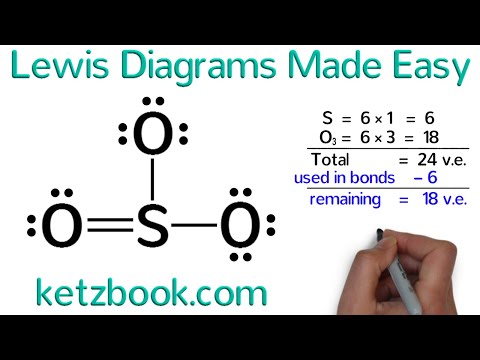
Sulfur: [Ne]3s²3p⁴ The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two. Krypton (Kr) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
(=6 valence electrons). To write the Lewis dot diagram for polonium, write the symbol for polonium, Po.
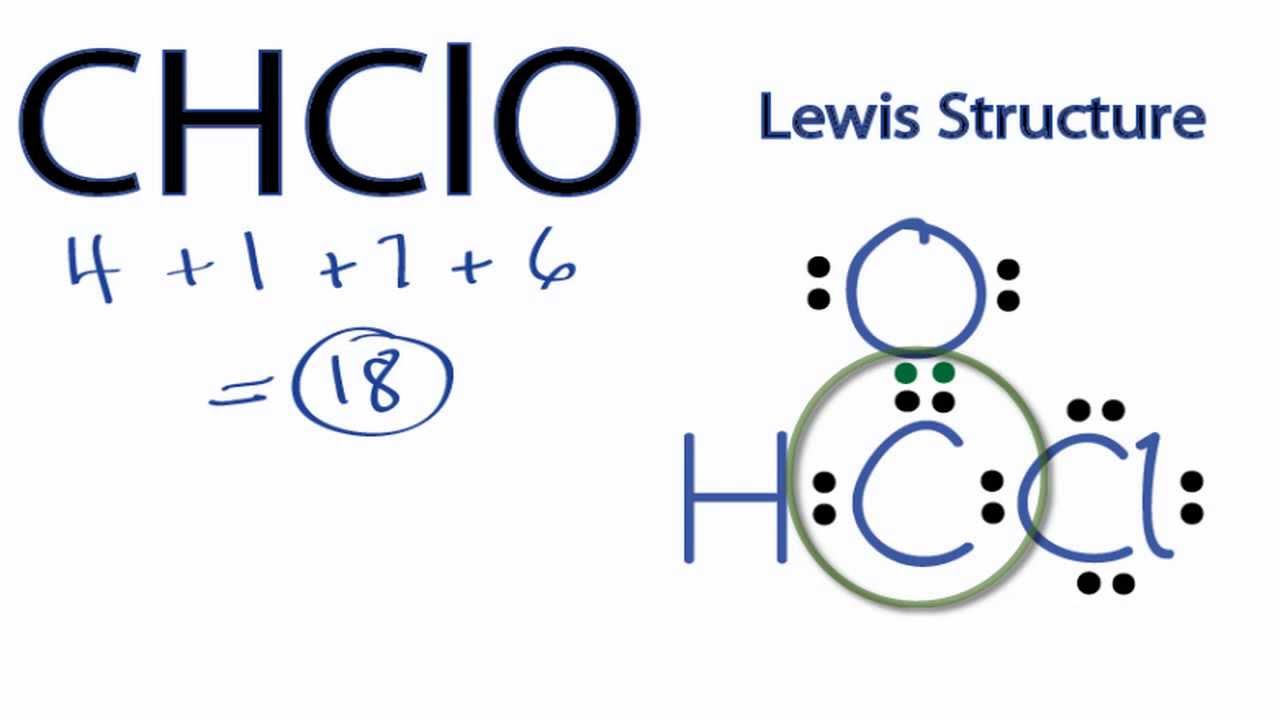
Then place one electron on each side of the symbol, then place another electron on two sides. You should have two electrons on two sides of the symbol, and one electron on two sides of the symbol.

The total number of electrons should be six. Nov 08, · Best Answer: The electron-dot structure can be used for any elements.
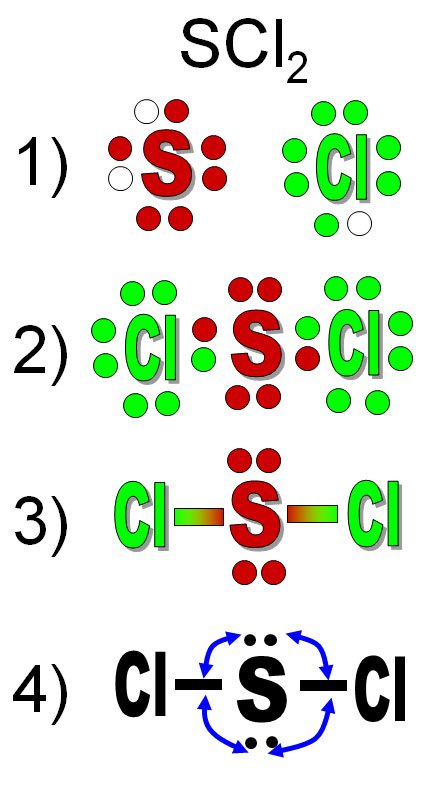
It just shows how electrons are distributed between bonds or number of valence electrons. The dot-structure is drawn by laying out the element symbol and putting dots (resembling valence electrons) around Status: Resolved.
Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.What is the electron dot diagram for kryptonWhat is the electron dot diagram for krypton