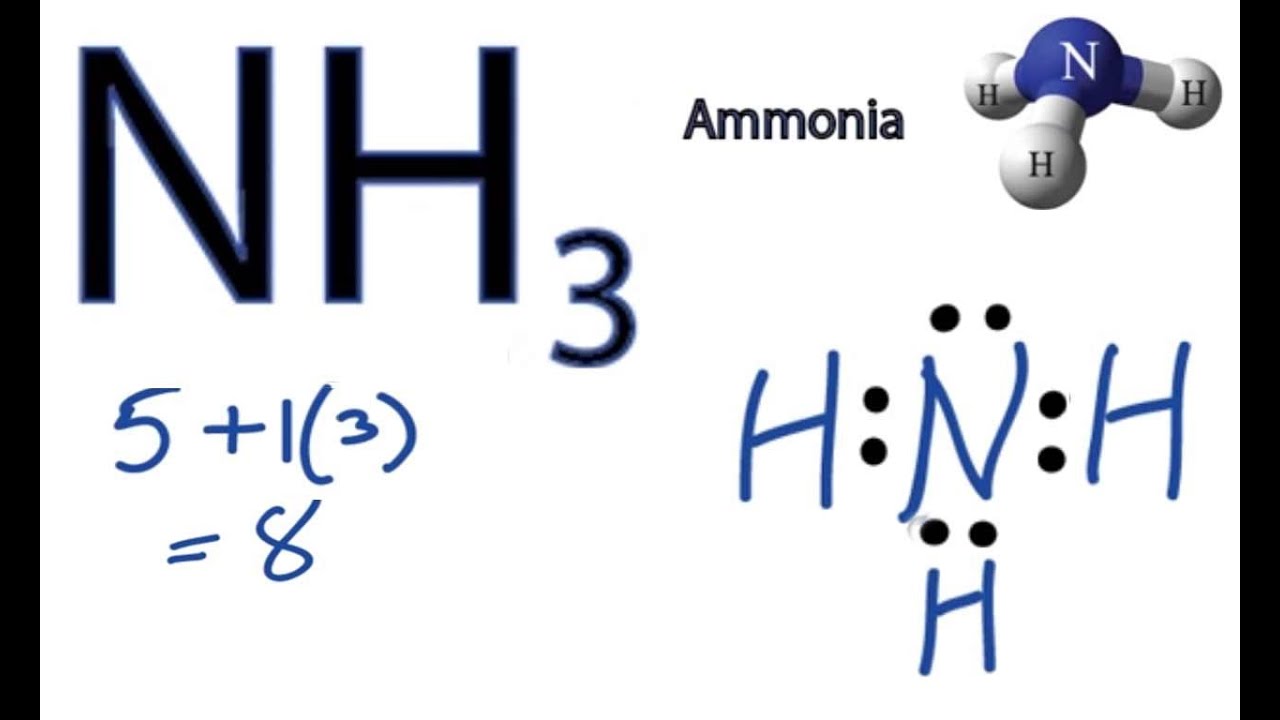
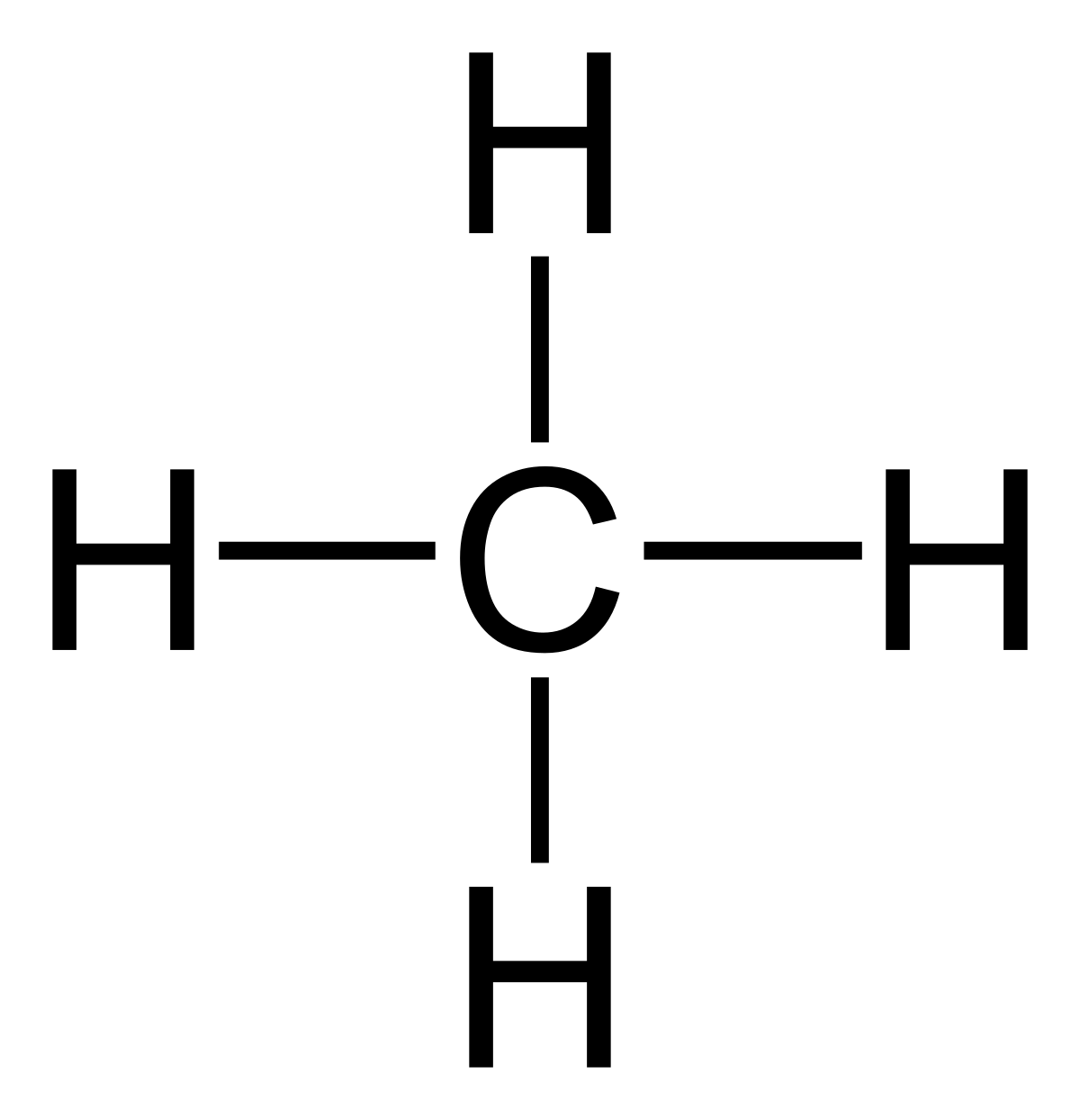
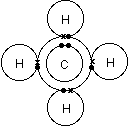
I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above.
The red one in the. The Lewis Dot Structure for CH4 is shown above.
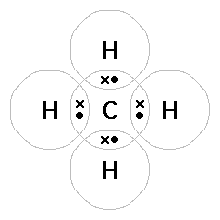
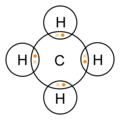
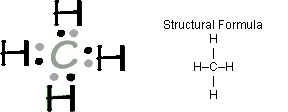
These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the . Find an answer to your question draw the electron dot structure of CH4.
A secondary school revision resource for AQA Additional GCSE Science about atomic structure and covalent bonding. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen).
For the O2 Lewis structure, calculate the total number of valence electrons for the O2 molecule. Get the free “Lewis structure” widget for your website, blog, WordPress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
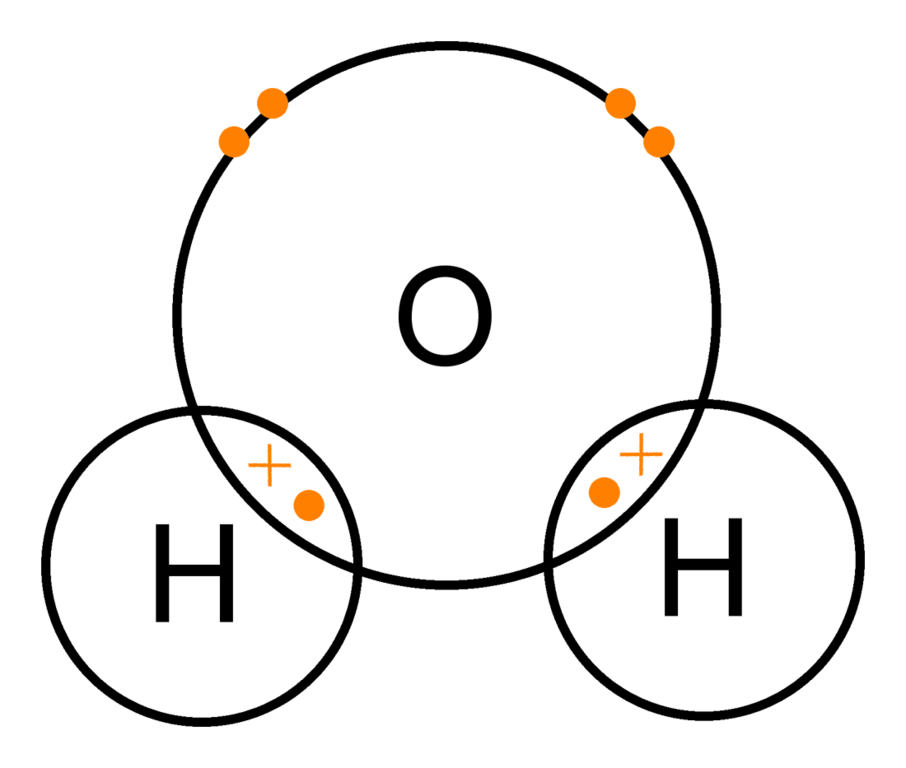
You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom The skeleton structure is H-O-H.
O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
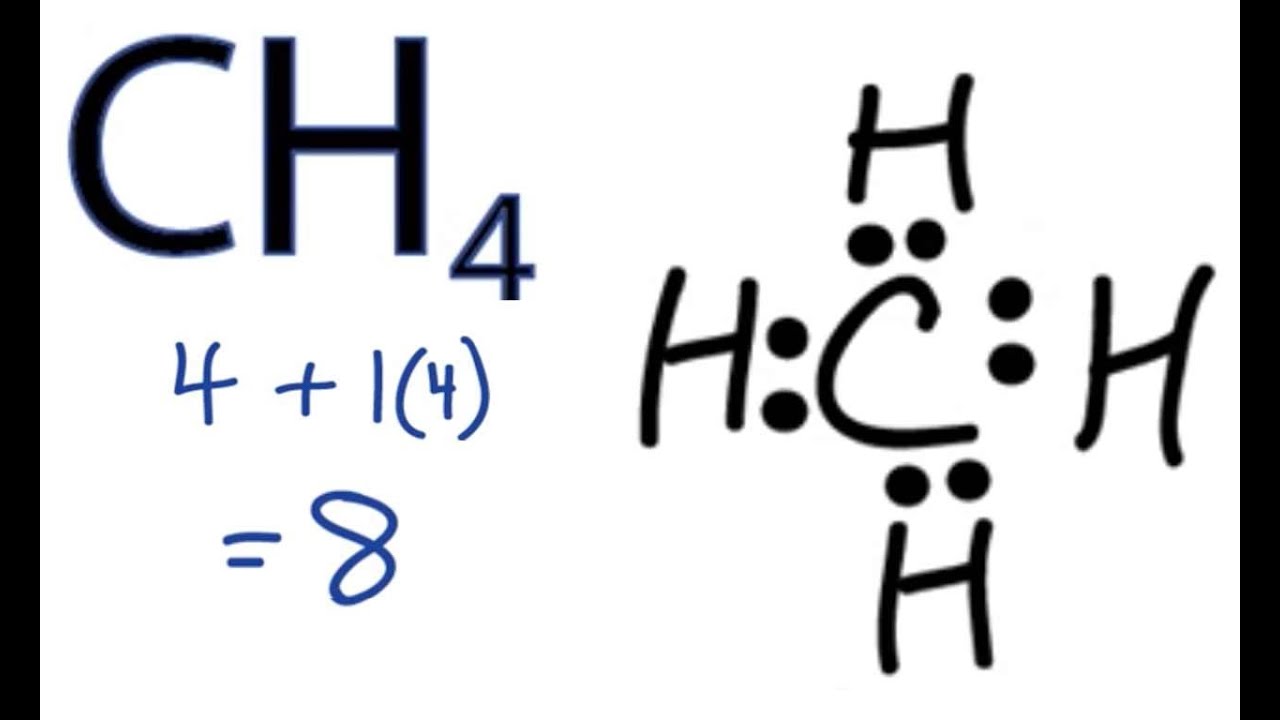
The trial structure is You have eight valence electrons in your trial structure, so it has the. Reach more of the million monthly visitors on Quora. Try our new broad targeting option, which optimizes ad delivery to reach people most likely to engage.
I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H’s in the Lewis.
Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane).
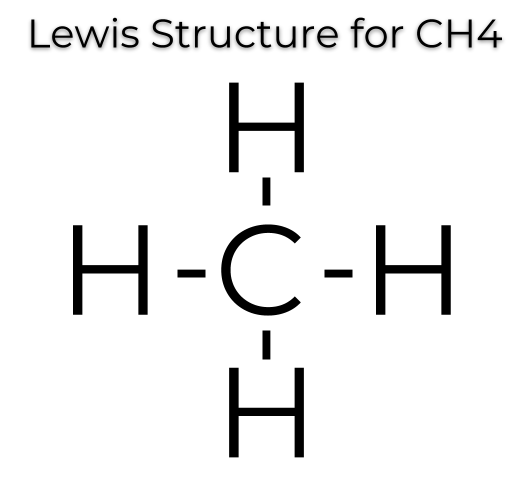
The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure.
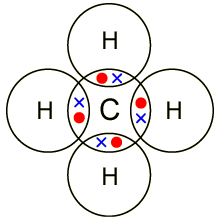
This is because they can share a maximum of two electrons.The Lewis Dot Structure for CH4 – MakeTheBrainHappyLewis Dot Structure for CH4 | Chemical Bonding | Electron Dot Structures