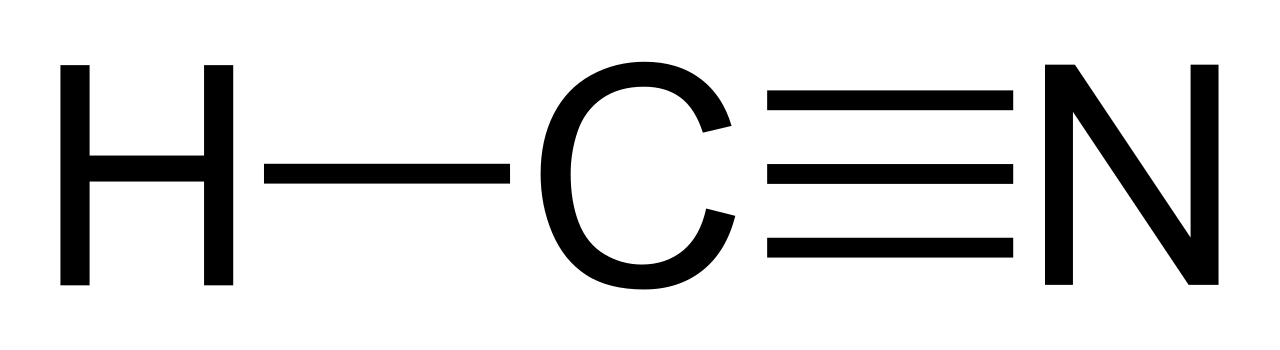
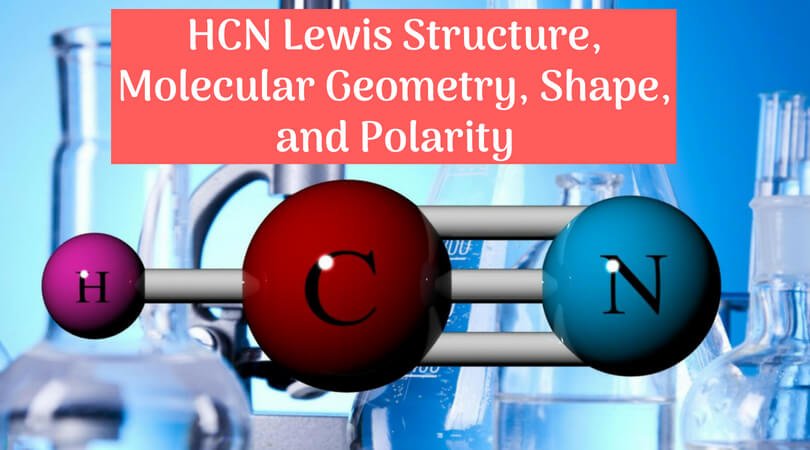
Lewis structure of HCN.

Step method to draw lewis structure of Hydrogen cyanide. Step 1: Find valence e- for all atoms. Add them together.
H C N Lewis structure of HCN. Step method to draw lewis structure of Hydrogen cyanide. Step 1: Find valence e- for all atoms.
Add them together. H C N In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs.
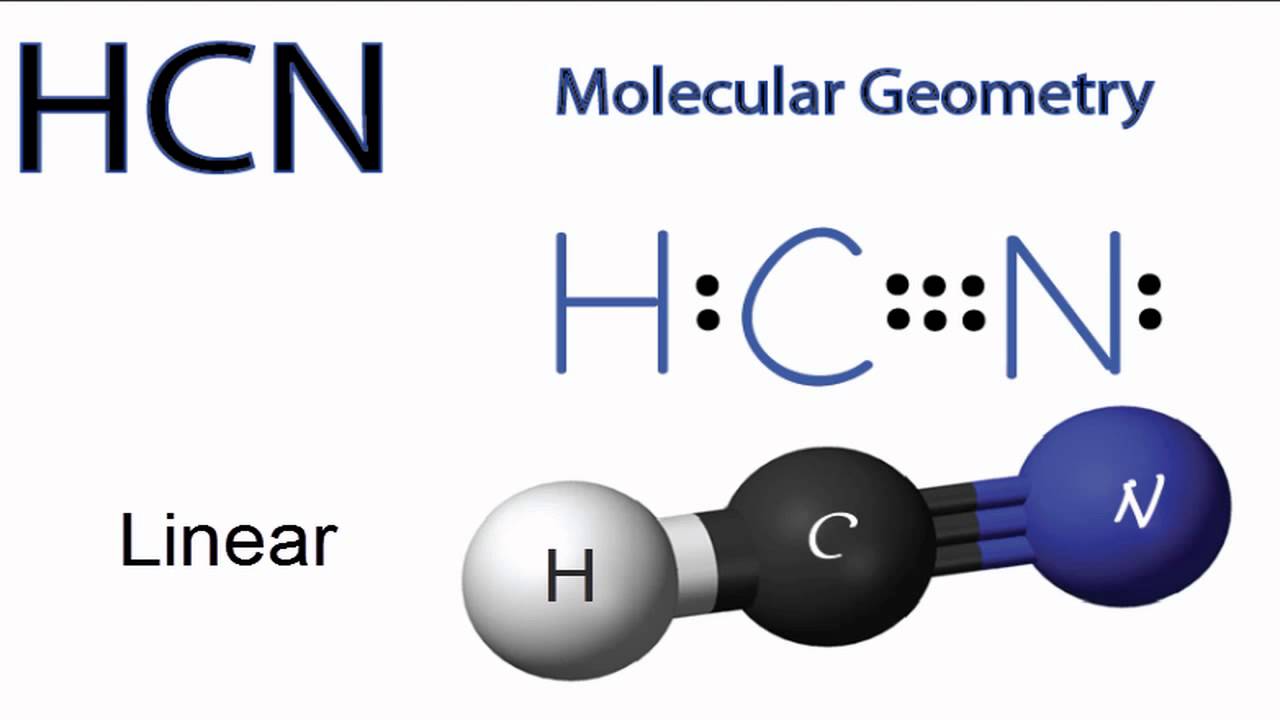
The carbon and nitrogen are bonded through. In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs. The carbon and nitrogen are bonded through.
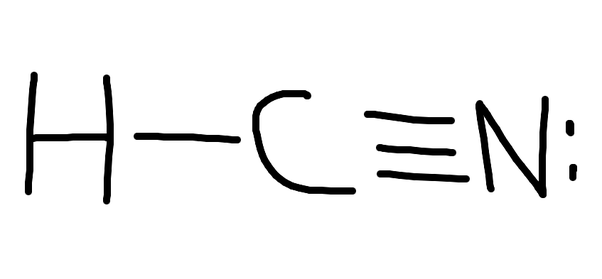
Learn to draw the Lewis structure of HCN & understand molecular geometry, shape, & polarity about the same by reading this article. Refer this.Drawing the Lewis Structure for HCN.
Viewing Notes: Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you’ll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms.
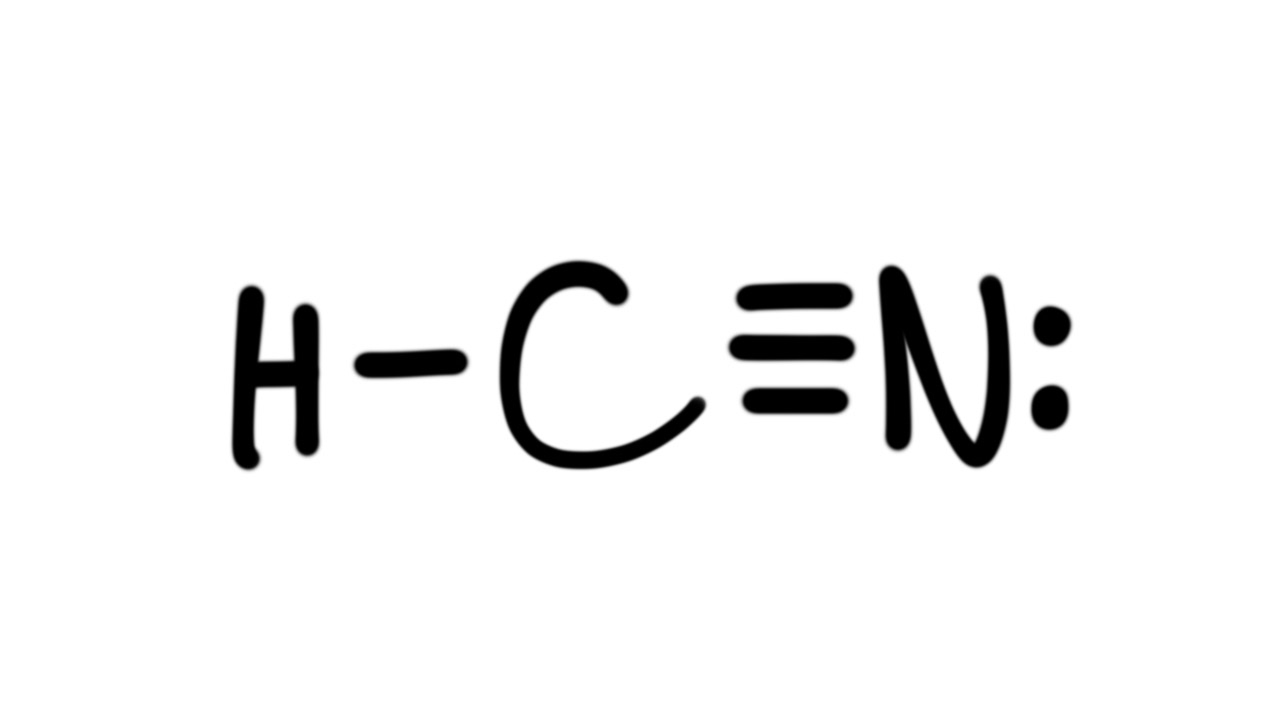
Sep 10, · Best Answer: C-N-H would be incorrect lewis structure because it shows that C is bonded to N which is bonded to H. The books version is correct, because everything should be bonded to C, as is the case in almost all C containing compounds. For example:Status: Resolved. The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple.
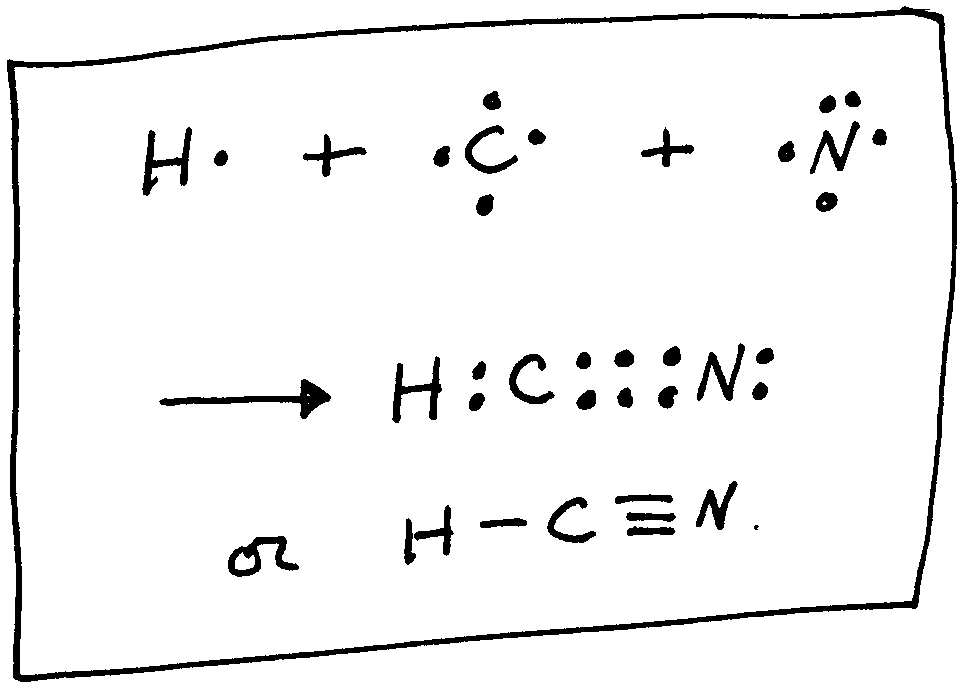
Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond the carbon atom to a single hydrogen atom. The nitrogen atom will have a lone pair placed on it.
Use information from step 4 and 5 to draw the lewis structure. Carbon goes in the schematron.org sure carbon and Nitrogen get 8 electrons to fulfil octet rule. Lewis dot structure of HCN.

Alternatively a dot method can be used to draw the lewis structure of BF 3. Calculate the total valence electrons in BF 3 molecule.
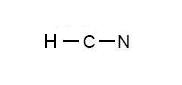
H:1 C:4 N Total= A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number .HCN Lewis Structure, Molecular Geometry, Shape, and PolarityWhat is the correct Lewis structure for HCN? | Yahoo Answers