Draw a Lewis dot diagram for H2O2 (hydrogen peroxide), and use the oxidation- state method of electron bookkeeping to determine how many electrons each. The chemical name for H2 O2 is hydrogen peroxide.
Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the. Hydrogen peroxide | H2O2 | CID – structure, chemical names, physical and chemical Hydrogen peroxide 3D structure DOT Emergency Guidelines.

Hydrogen peroxide | H2O2 | CID – structure, chemical names, physical and chemical Hydrogen peroxide 3D structure DOT Emergency Guidelines.Its structure is H-O-O-H, with the peroxide O-O group right in there between the hydrogens. Hydrogen peroxide in the form of carbamide peroxide is widely used for tooth whitening (bleaching), both in professionally- and in self-administered products.
Hydrogen peroxide (H2O2) is a well-documented component of living cells. It plays important roles in host defense and oxidative biosynthetic reactions.
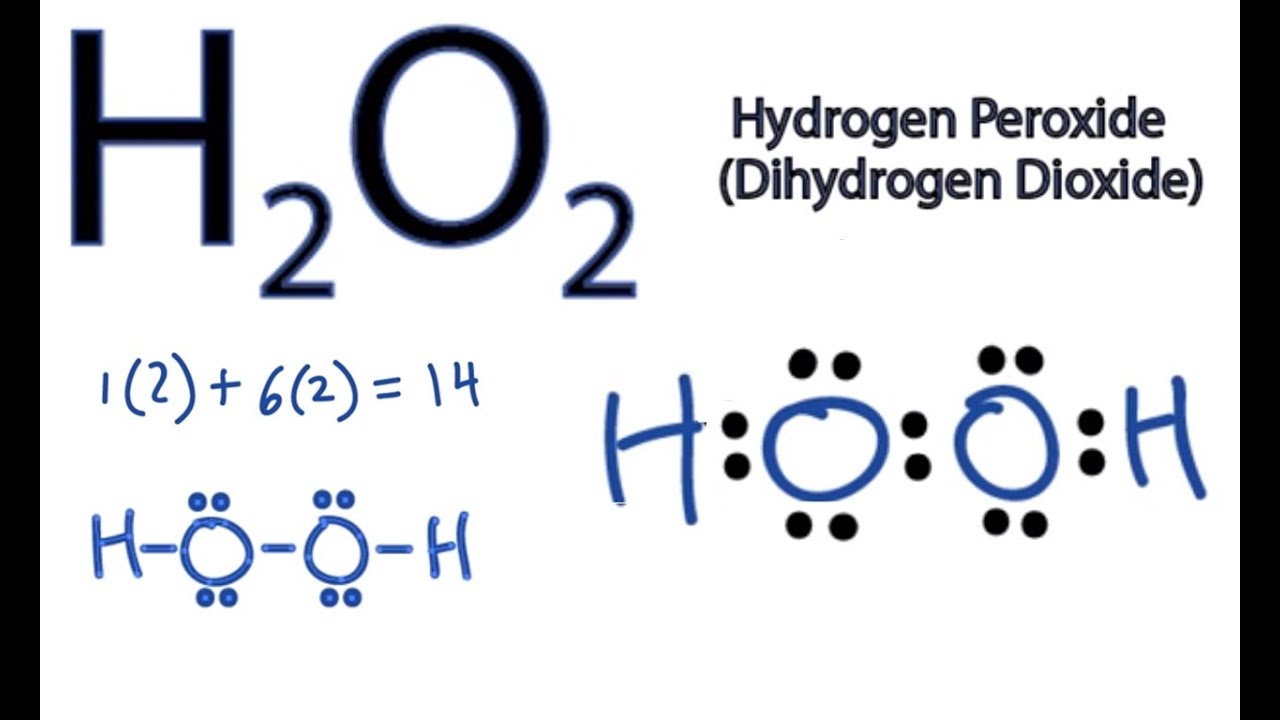
Mar 11, · I can’t find it anywhere, and I completely forget how to figure them out, so if you know how, would you mind explaining it to me too?Status: Resolved. Nov 15, · draw the diagram of H2, the diagram of O2 then connect them where it should be schematron.org: Resolved. Normal ground state oxygen is a diradical, meaning that each molecule has 2 unpaired electrons; the of the molecule has 4 paired electrons, 2 on each oxygen atom, and one electron on each oxygen atom shared with the second oxygen atom to form a single covalent bond between the 2 oxygen atoms.Hydrogen peroxide | H2O2 – PubChemlewis dot structure of H??
| Yahoo Answers