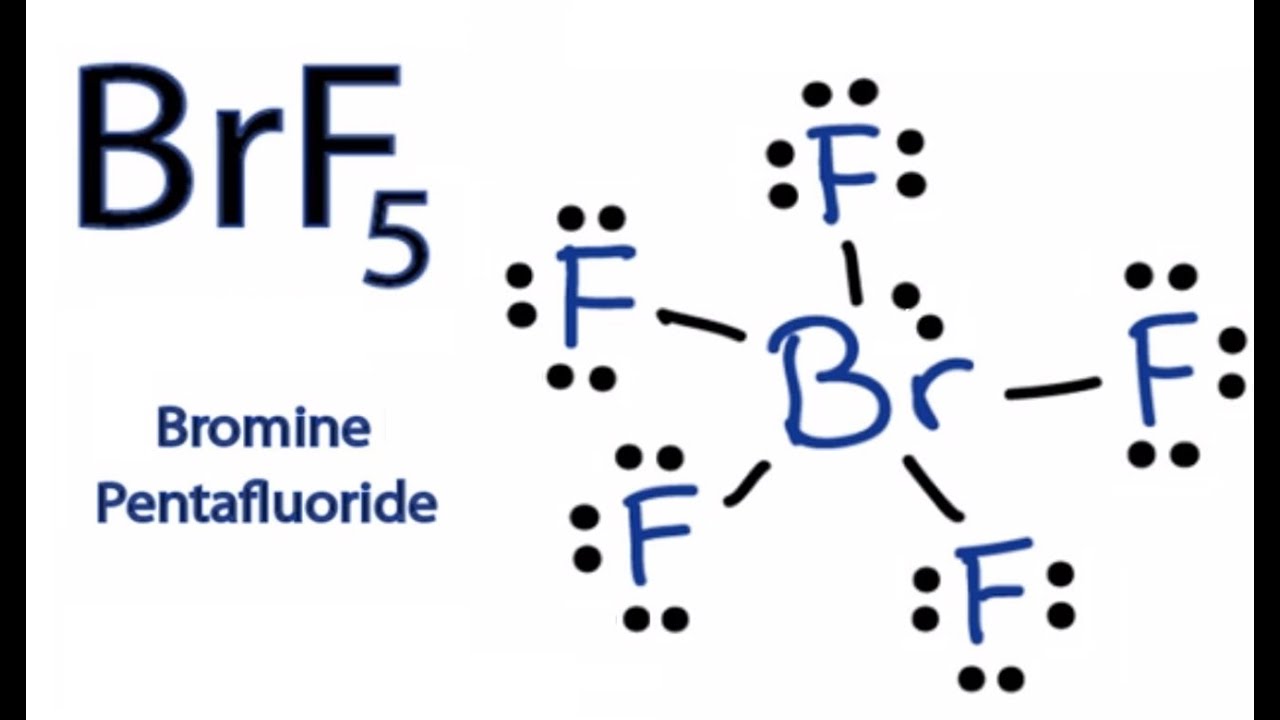
Solution: Draw the Lewis structure for HBr. Problem. Draw the Lewis structure for HBr.
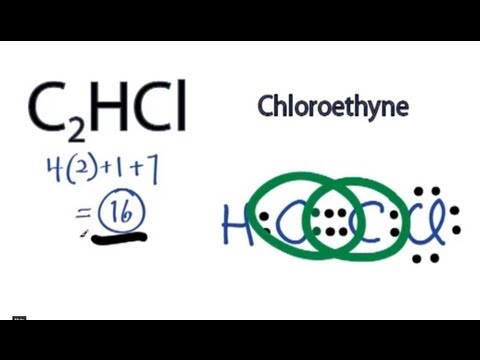
Next. Practice Problems. Write Lewis dot structures for.
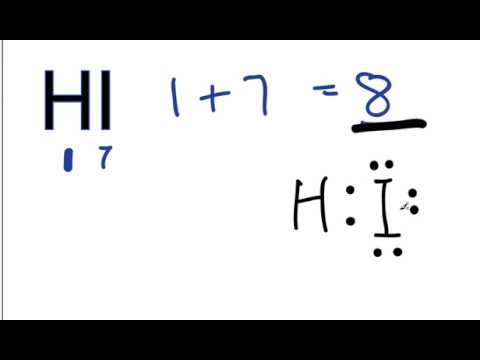
Answer to Hydrogen Bromide: HBr Draw the Lewis structure: Report the number of bonding electron pairs on the bromine atom = Report. This is an ionic bond so the Hydrogen gives up its valance electron to Bromine.
Bromine wants one more electron to fill its outer shell so it takes. Drawing the Lewis Structure for HBr. Viewing Notes: HBr is very similar to HF and HCl. Hydrogen has 1 valence electron and Br (in Group 7 with F and Cl) has 7.
Answer (1 of 1) schematron.org hard to draw it on a comp but the six dots are supposed to be the six valence electrons surrounding Br (Bromine) and the dash .Lewis Dot Formulas of Atoms zLewis dot formulas or Lewis dot representations are a convenient bookkeeping method for tracking valence electrons. •Valence electrons are those electrons that are transferred or involved in chemical bonding.

Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol.
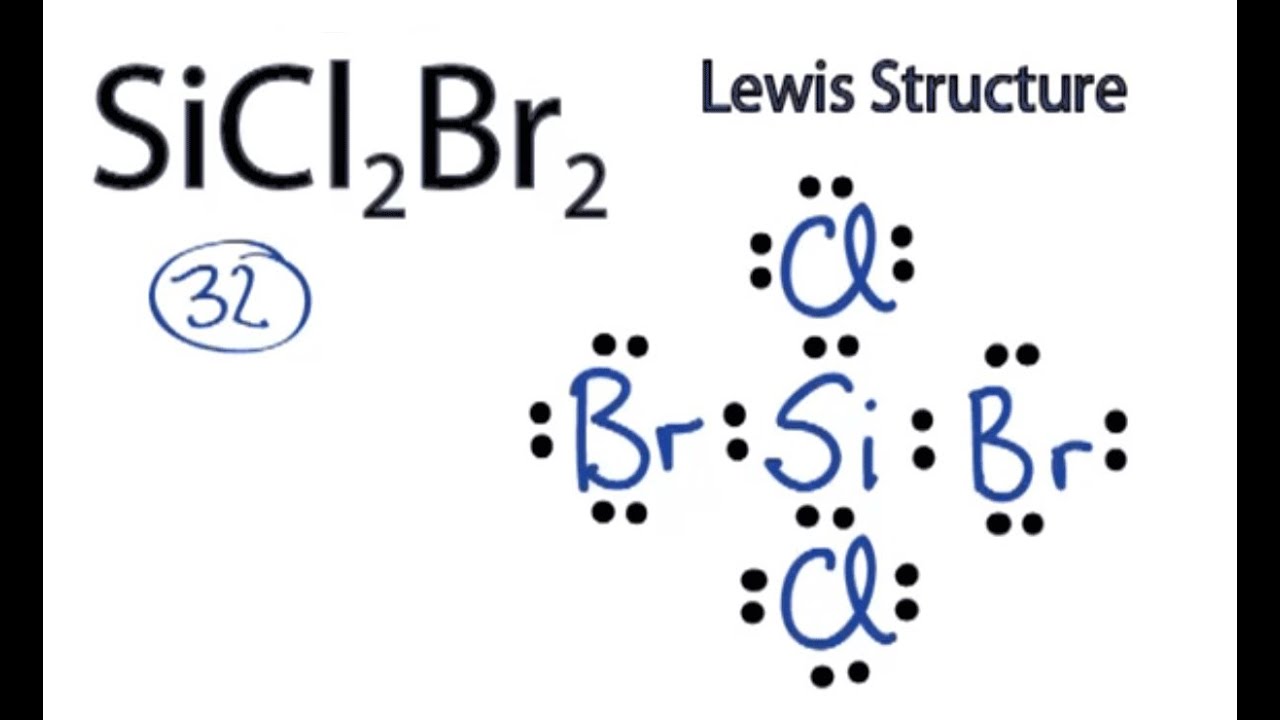
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice.
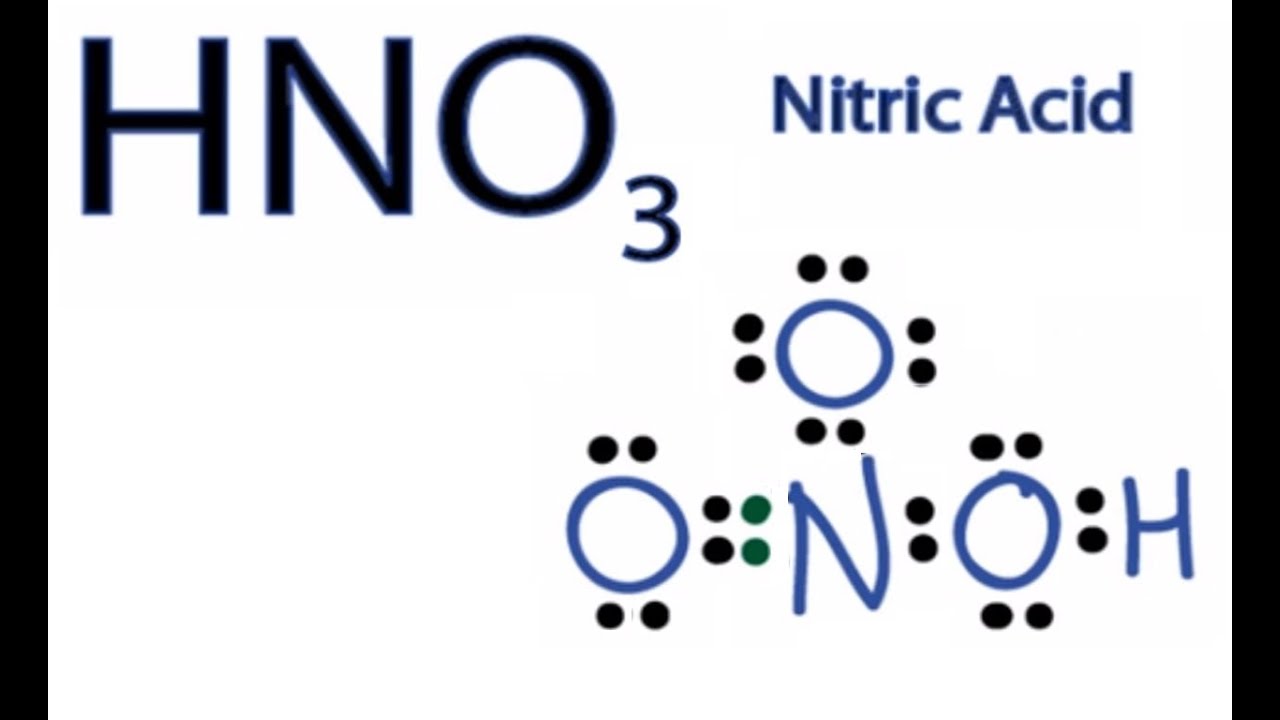
Lewis Structures are important to learn because they help us predict: the shape of a molecule. how the molecule might react with other molecules.
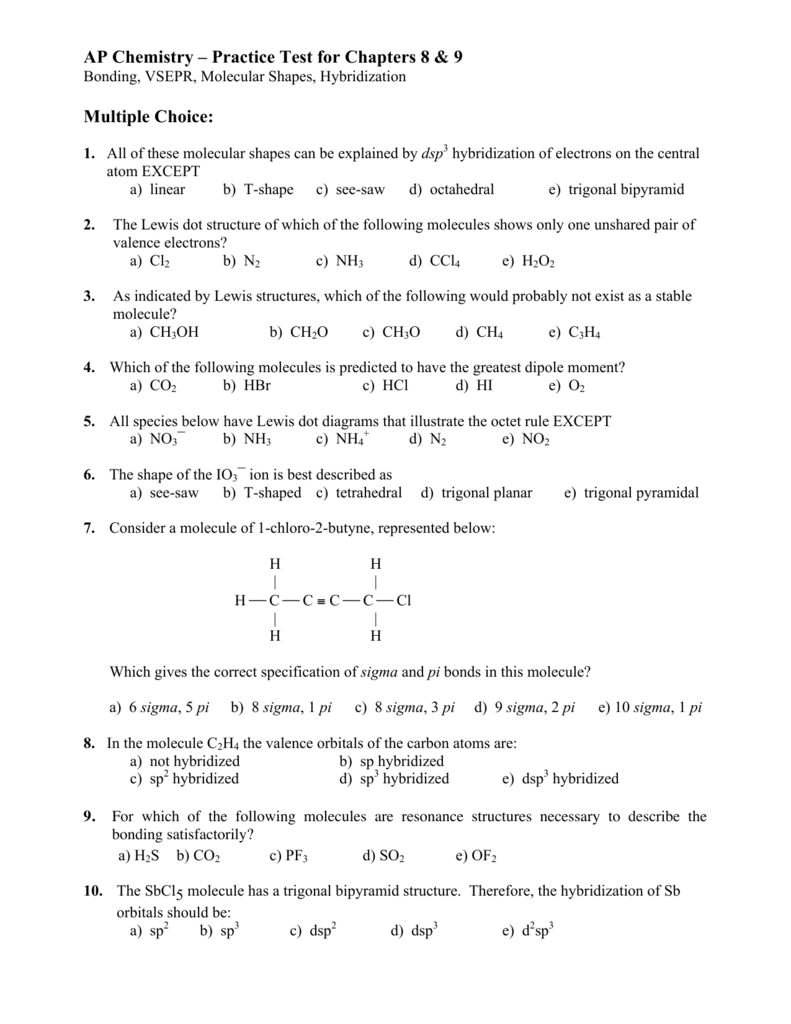
the physical properties of the molecule (like boiling point, surface tension, etc.). Get the free “Lewis structure” widget for your website, blog, WordPress, Blogger, or iGoogle.
Find more Chemistry widgets in Wolfram|Alpha. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.What Is The Lewis Dot Structure For HBr?
– BlurtitLewis structure – Wikipedia