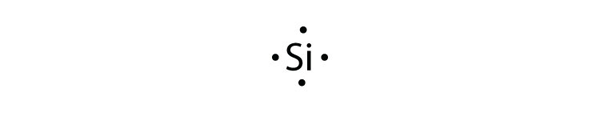
To answer your question, you would draw the resonance structure of each Lewis Dot Structure and then apply Formal Charge calculation to. Column 1A 1 valence electron The first symbol in the column is H .

electrons and draw the Lewis dot structure. 1. Barium 6.
Carbon. 2.
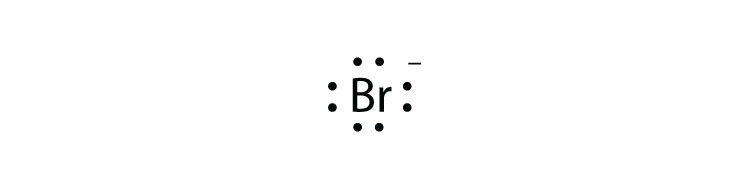
Tin 7. Krypton.
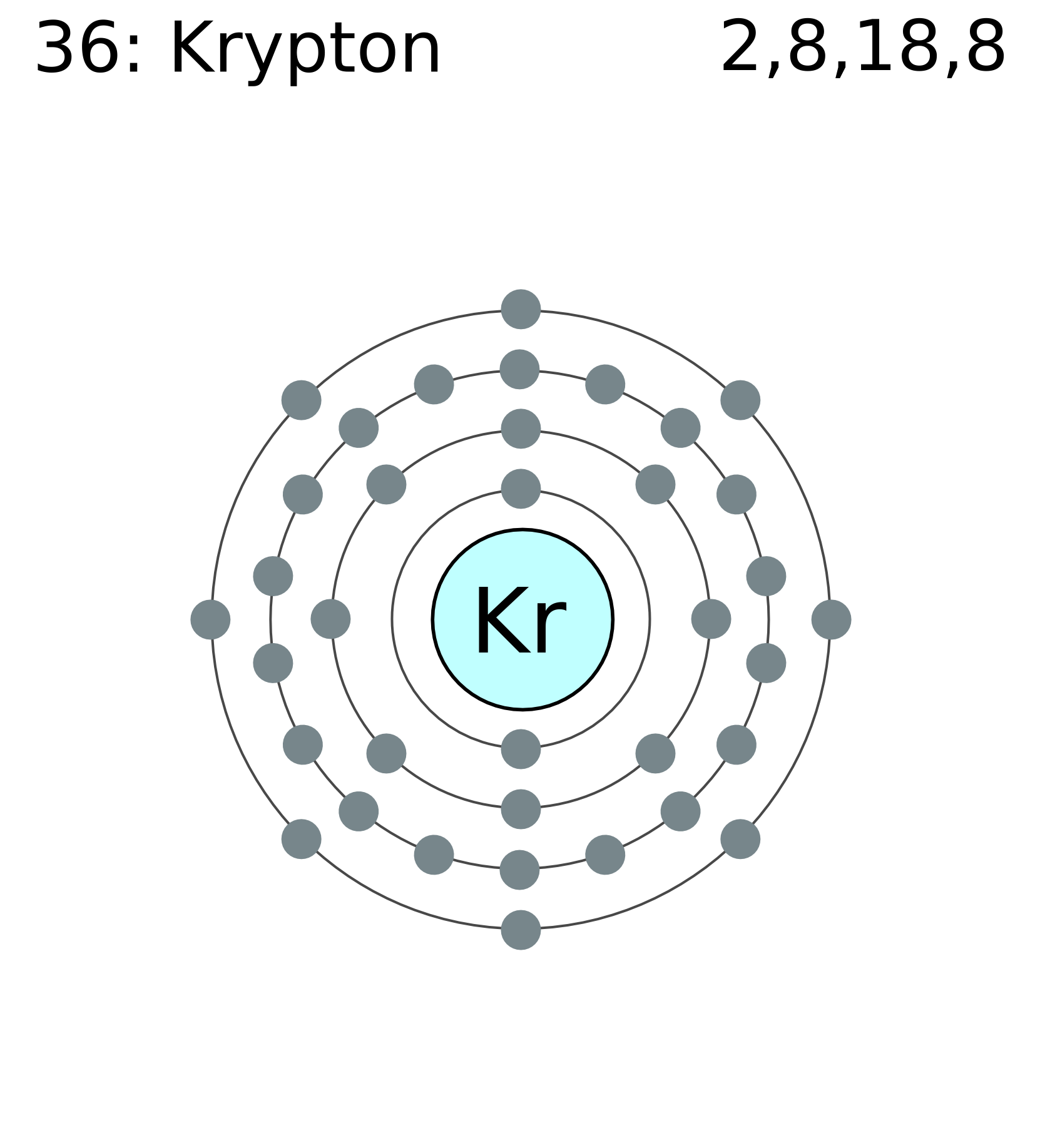
3. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are . Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their. Column 1A 1 valence electron The first symbol in the column is H .
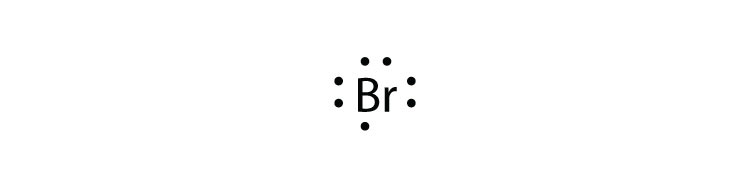
electrons and draw the Lewis dot structure. 1. Barium 6.
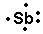
Carbon. 2. Tin 7. Krypton.

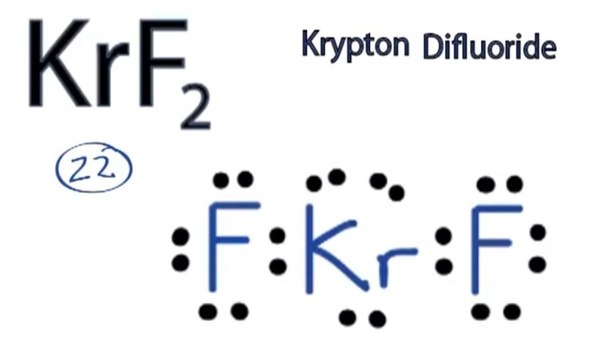
3. Krypton(Kr) is in Group 18 (sometimes called Group VIII or 8A or When you draw the Lewis structure for Krypton you’ll put eight “dots” or.Aug 04, · Krypton difluoride, or KrF 2, has the Lewis structure of a krypton atom with 3 lone pairs, single bonded to two fluorine atoms, each also containing 3 lone pairs.
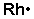
Krypton has 8 valence electrons, whereas fluorine contains 7 valence electrons. A lone pair .
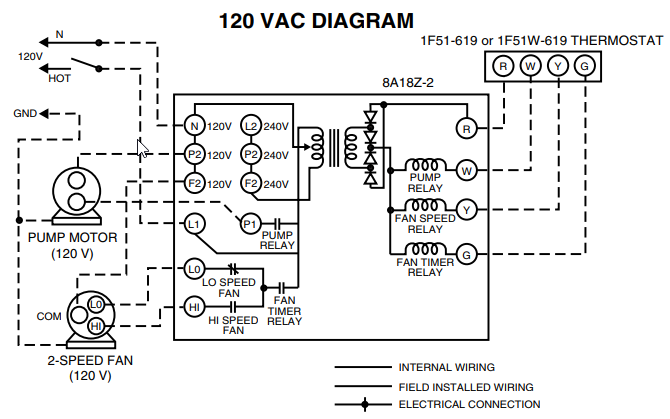
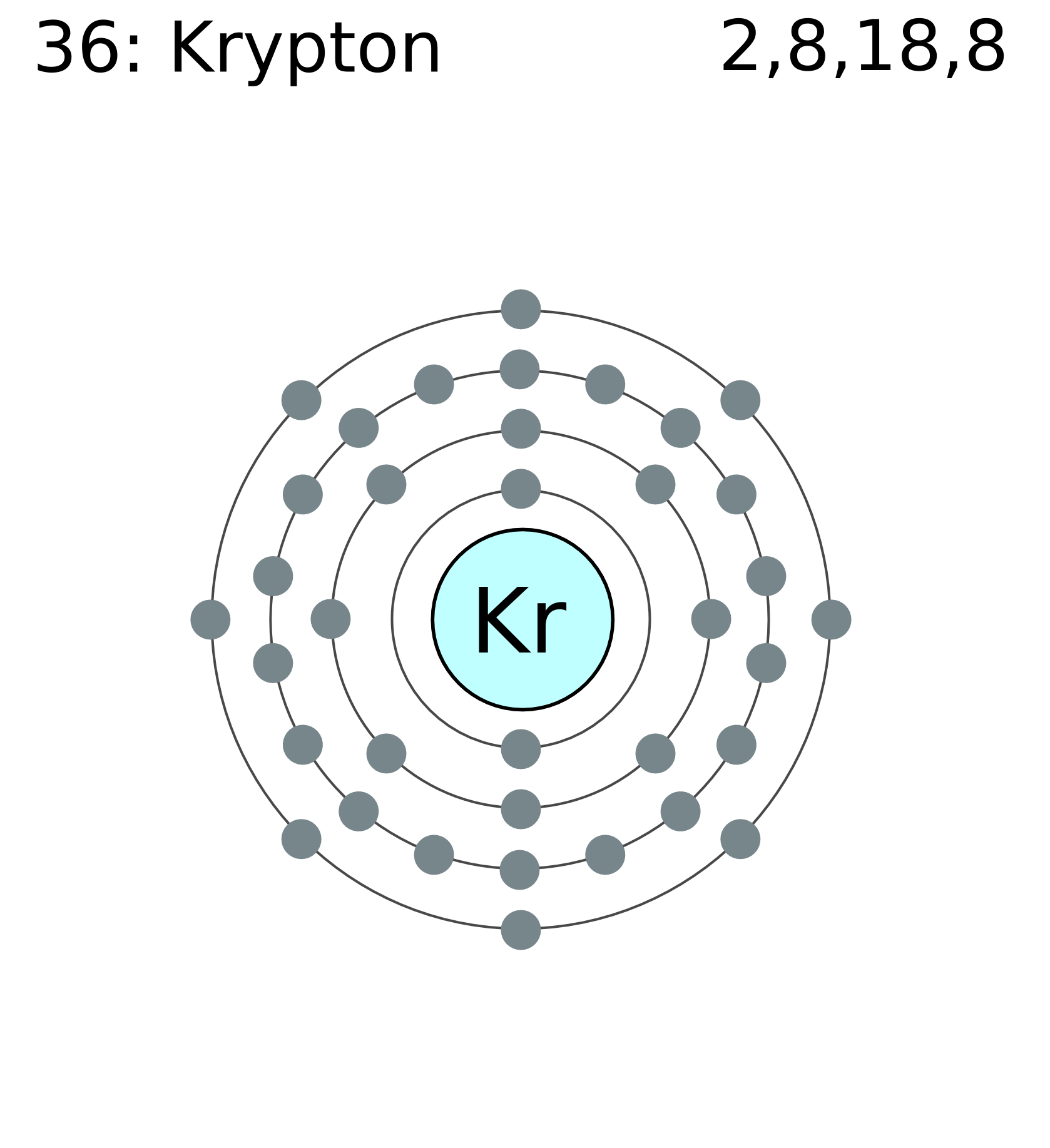
Krypton 36 Lewis (Dot) Diagram: Even atoms with more than 20 electrons are easy. Example 4: Krypton Krypton is in group 8A.
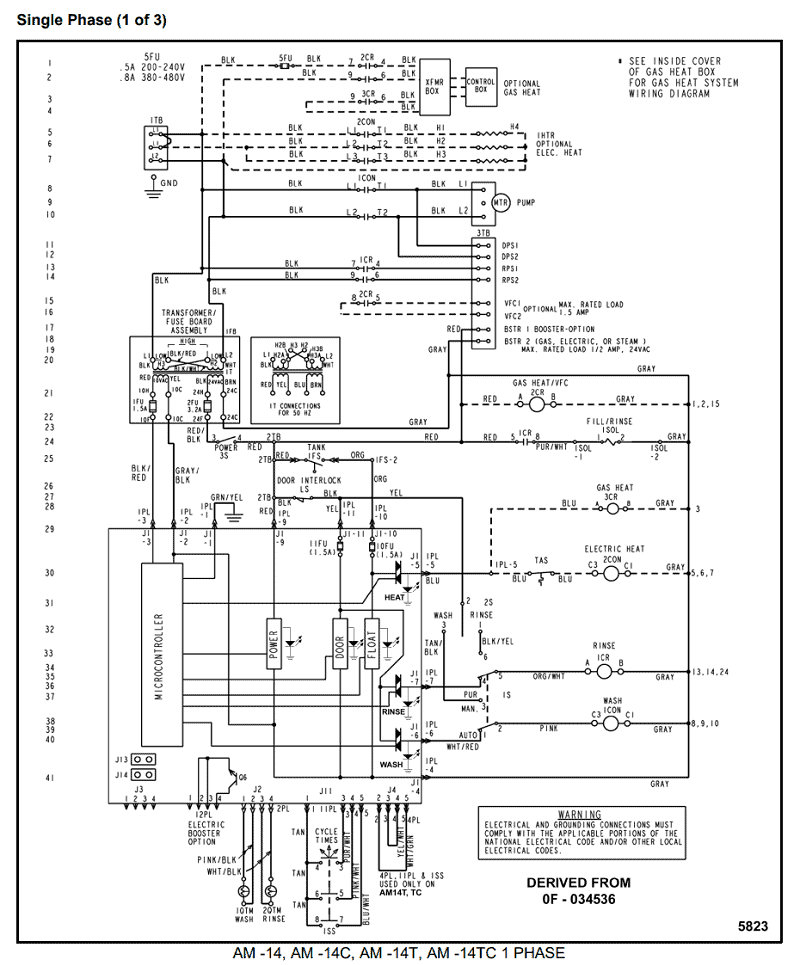
Krypton atoms have 8 valence electrons. Ra Ra Radium 88 Lewis (Dot) Diagram: Example 5: Radium Radium is in group 2A.
Radium atoms have 2 . Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom.

Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Krypton difluoride, KrF 2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered.

[2] It is a volatile, colourless solid. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.What is the correct electron dot diagram for krypton krHow do you draw a Lewis dot structure of Kr