Video created by Georgia Institute of Technology for the course “Material Processing”. This course picks up with an overview of basic thermodynamics and .
However, unlike the monotectic system, the syntectic one may exhibit a symmetric phase diagram which drastically simplifies the solidification problem. monotectic point, monotectic reaction isotherm, hypomonotectic, and hypermonotectic. the phase diagram shows a dome-shaped region within.
Lecture Introduction to Monotectic Phase Diagram. Phase Diagrams in Materials Science and Engineering. Loading Unsubscribe from.
We also investigate monotectic solidification using the phase-field method. . This is different from the monotectic phase diagram, where.Monotectic Phase diagram Another three phase invariant reaction that occurs in some binary system is monotectic reaction in which a liquid transforms to another liquid and a solid.
L 1 L 2 +. Two liquids are immiscible like water and oil over certain range of compositions.
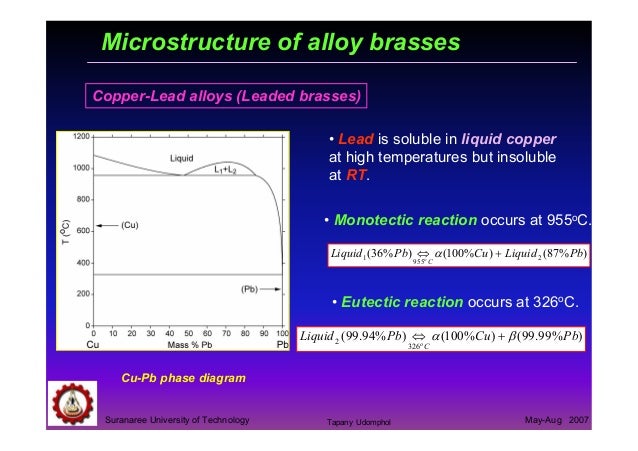
Cu-Pb system has a monotectic at 36%. phase diagram.
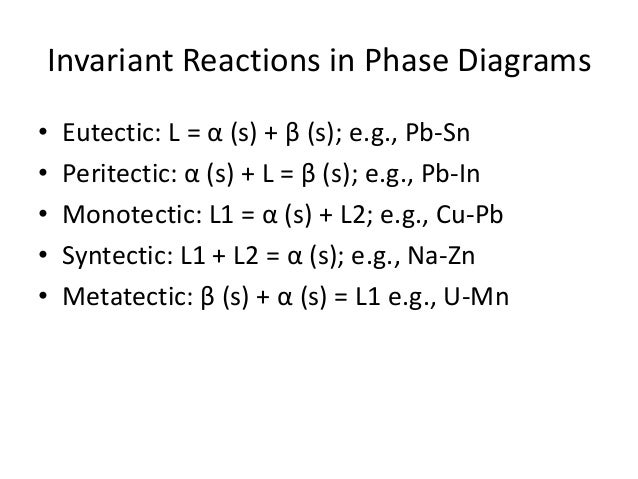
PHASES A phase is a homogeneous portion of a system with uniform physical and chemical characteristics, in principle separable from the rest of the system. monotectic reaction occurs: Below T 3, the liquid phase separates into two liquids of different comp.
The first module deals with phase diagrams – charts that tell us how a material will behave given a certain set of variables such as temperature, pressure, and composition. You will learn how to interpret common and complex phase diagrams and how to extract useful information from them.
characteristics. Phase boundaries separate two distinct phases.
A single phase system is called homogeneous. A system with two or more phases is called heterogeneous.
Phase Diagram –a graphic representation showing the phase or phases present for a given composition, temperature and pressure. The two immiscible liquid phases in equilibrium with a single liquid phase have been observed during the phase diagram study of an organic analog of a metal–nonmetal system involving pentachloropyridine (PCP)–succinonitrile (SCN).
The phase equilibrium shows the formation of a monotectic and a eutectic, with large miscibility.Liquid and Solid Standard States – ppt downloadEutectic system – Wikipedia