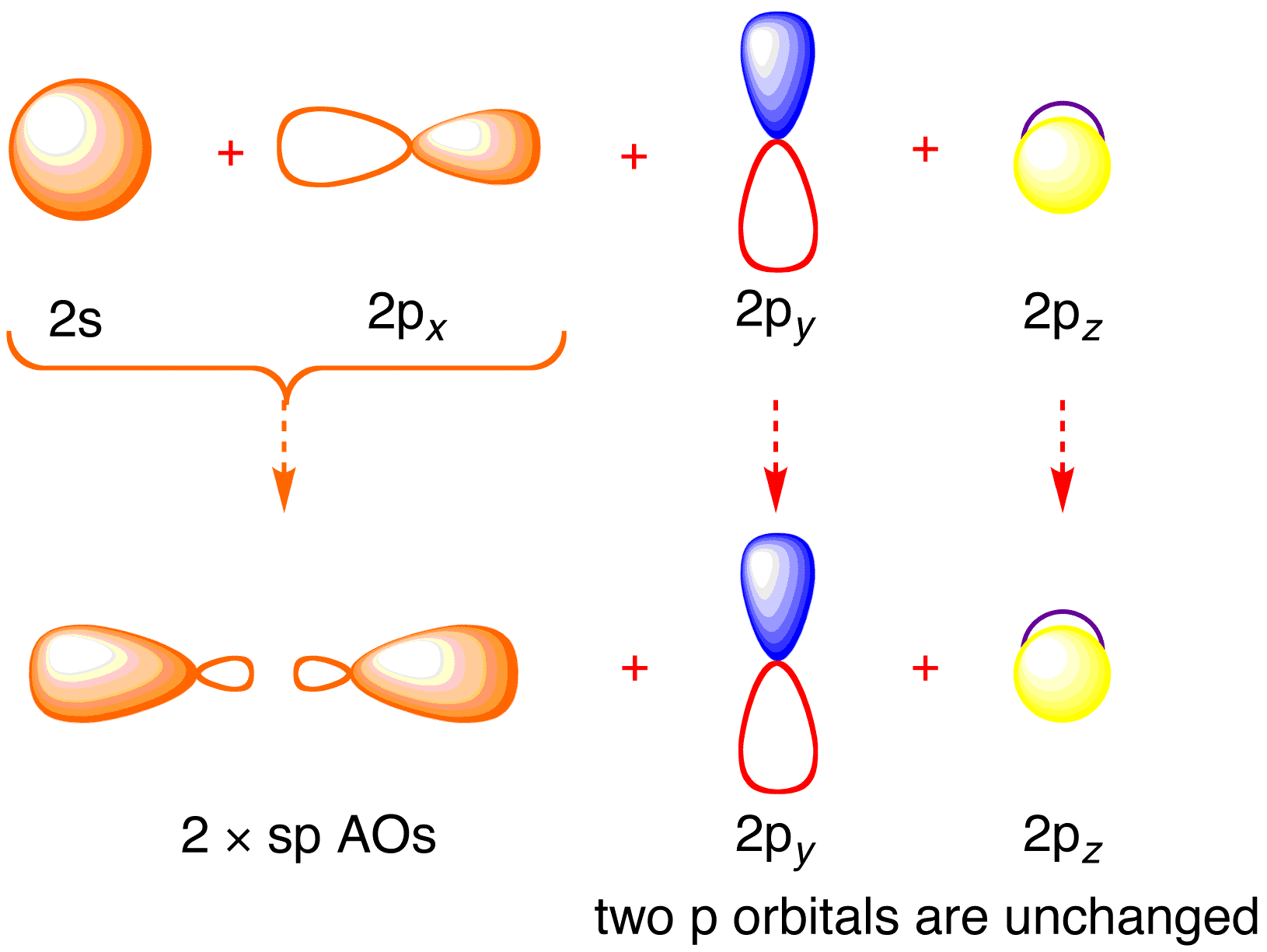
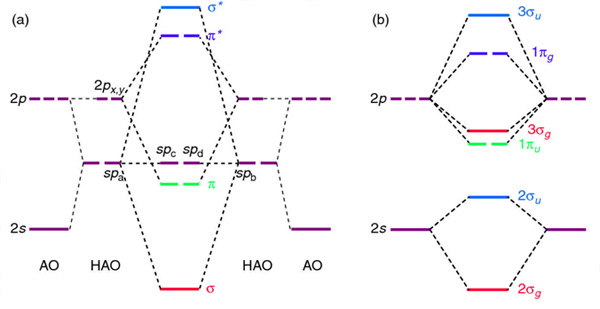
Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid . The carbon atoms in ethyne use 2sp hybrid orbitals to make their sigma bonds.
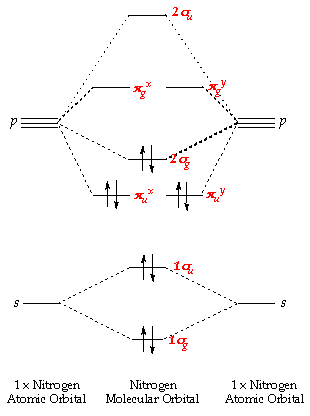
After. Acetylene (ethyne) is perhaps the most interesting example. With a triple bond between carbons, there must be two orthogonal π-bonds.
These are pretty easy to. Alkynes: Molecular Orbital Model.

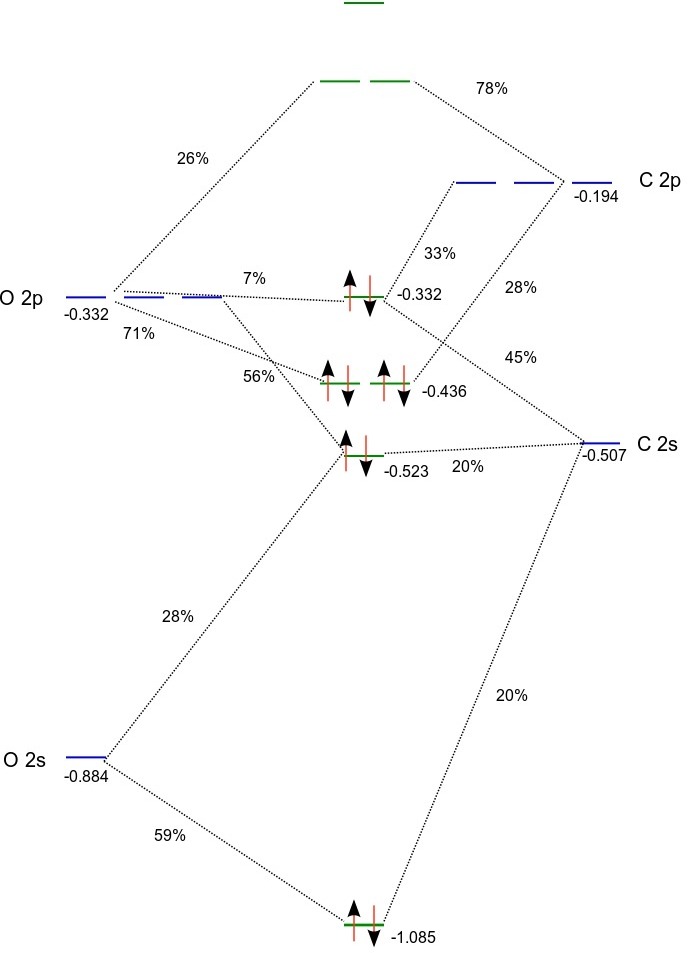
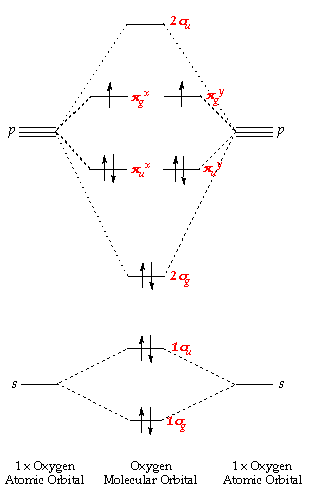
Fig MO Diagram of the C-C bond of acetylene. Molecular orbital theory (MO theory) combines atomic or hybrid orbitals.
An explanation of the bonding in ethyne (acetylene), including a simple view of hybridisation. In the diagram each line represents one pair of shared electrons. which are pointing towards each other now merge to give molecular orbitals. Bonding orbitals in Ethyne (Acetylene) sp.
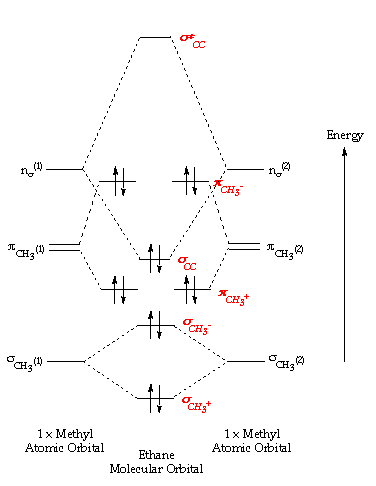
sp hybrids. acetylene orbitals.
Jmol. _Canvas2D perpendicular. Explore bonding orbitals in other small molecules.Simple Molecular Orbitals – Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom.
When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Alkynes: Molecular and Structural Formulas The alkynes comprise a series of carbon- and hydrogen-based compounds that contain at least one triple bond.
This group of compounds is a homologous series with the general. molecular formula of C n H 2 n, where n equals any integer greater than one. The simplest alkyne, ethyne.
The acetylene (C 2 H 2) has sp-hybridization and it is explained as the two carbon atoms undergo mixing of one s and one p-orbitals to form two sp-hybridized orbitals and the sp-hybridized orbital of the C-atoms make a C-C sigma bond while the other sp-hybrid orbital of each C-atom overlaps with the s-orbital of one H-atom to form a C-H sigma bond. In ethyne molecule, each carbon atom is Sp-hybridized.
Due to Sp-hybridization each carbon atom generates two Sp-hybrid orbitals. In this way there exists four Sp-orbital in ethyne.
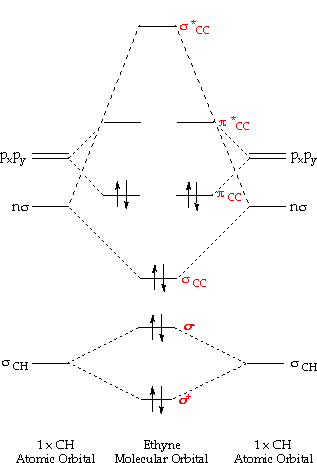
Two degenerate sp orbitals result. Each carbon atom in ethyne, therefore, has two sp orbitals and two unhybridized p orbitals (Figure ). One of the sp orbitals of one carbon in ethyne overlaps an sp orbital of the other carbon to form a carbon–carbon σ bond.\(sp\) Hybrid Orbitals and the Structure of Acetylene – Chemistry LibreTextsBonding in Ethyne: A Triple Bond | Organic Chemistry