Problem 44AP: Draw energy diagrams for exergonic reactions that meet the following descriptions:(a) A slow reaction that has a small free-energy change(b) A.
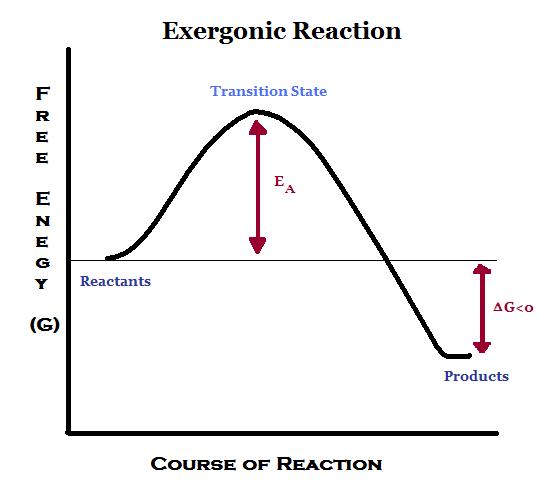
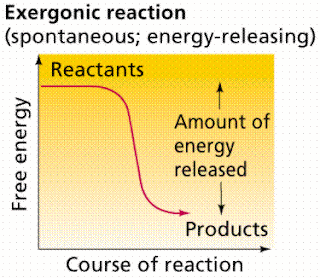
Even energy-releasing (exergonic) reactions require some amount of energy The activation energy shown in the diagram below is for the forward reaction. Download scientific diagram | 5: An exergonic reaction (such as cellular respiration) is a reaction that loses energy during the process of the reaction. An energy diagram for an exergonic or spontaneous reaction is shown to the right.
The energy level of the products is lower than the energy level of the. The Gibbs free energy graph shows whether or not a reaction is spontaneous– whether it is exergonic or endergonic.
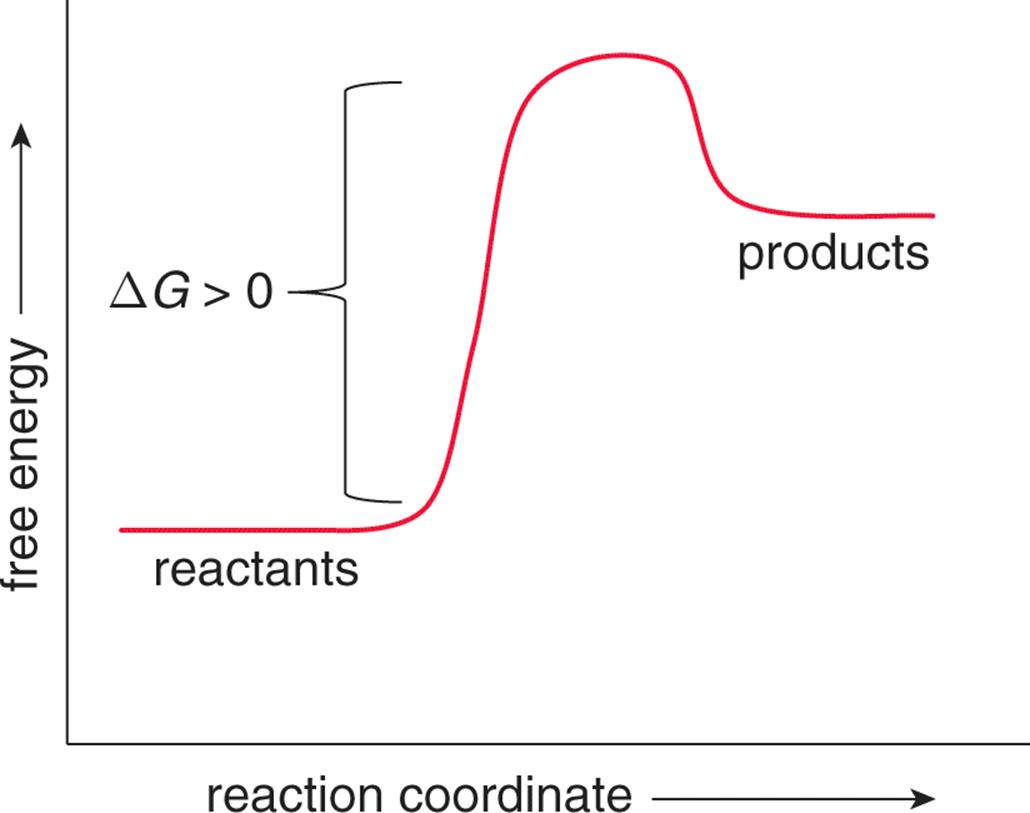
ΔG is the change in free energy. Generally .Endergonic vs Exergonic For the purpose of chemistry, the universe is divided into two parts.
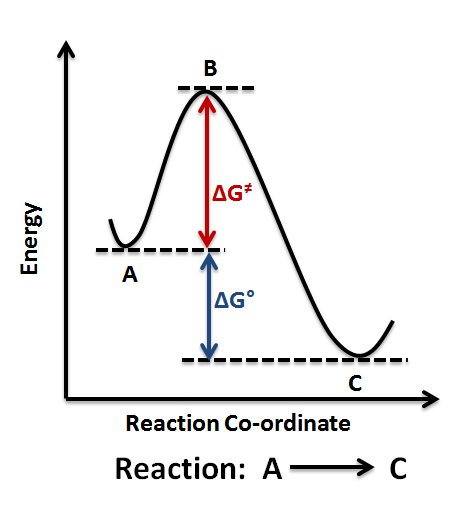
The part in which we are interested is called a system, and the rest is called the surrounding. A system can be an organism, a reaction vessel or even a single cell. Exergonic and endergonic qualifications only apply for Gibbs’ free schematron.orgpy applies to potential energy diagrams..
Endergonic just means that #DeltaG_”rxn” > 0#.So, the Gibbs’ free energy of the products is higher than the Gibbs’ free energy of the reactants. An energy hill diagram provides a visual display that shows whether a reaction is exergonic or endergonic.

The diagram includes two axes, time at the bottom and the total energy of the chemical solution on the side. An energy diagram for an exergonic or spontaneous reaction is shown to the right.

The energy level of the products is lower than the energy level of the reactants. Energy is released in this reaction.
The amount of energy released during the reaction is termed ΔG, which is less than zero. In chemical thermodynamics, an endergonic reaction (also called a heat absorb nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and energy is absorbed.
In layman’s terms, the total amount of energy is a loss (it takes more energy to start the reaction than what.Free energy | Endergonic vs exergonic reactions (article) | Khan AcademyEndergonic reaction – Wikipedia