Answer to draw the lewis dot structure for CH3CN.

Please help!. On the other hand, Lewis structures, also called Lewis-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and.
Structure, properties, spectra, suppliers and links for: Acetonitrile, Ethanenitrile, , MeCN, NCMe, CH3CN. ShowMe is an open learning community featuring interactive lessons on a variety of topics.

Drawing the Lewis Structure for CH3CN. Viewing Notes: There are a total of 16 valence electrons in the CH3CN Lewis structure.
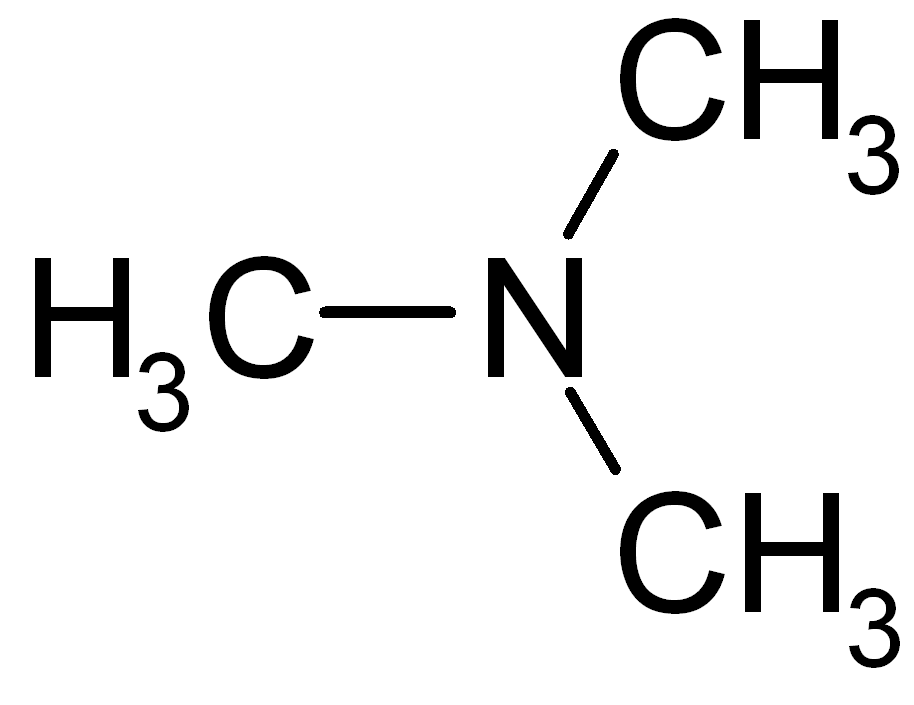
The CH3CN chemical formula.The Lewis structure of CH3OCH3 is dimethyl ether, the simplest ether in existence. The structure of the organic compound contains a total of 20 valence electrons. In the Lewis structure, the central oxygen atom is bonded to two carbon atoms, which have little or no electronegativity.
For the CH3CN Lewis structure, calculate the total number of valence electrons for the 2 years ago. SpaceChem – acetonitrile, benzole (CH3CN – C6H6, PhH) 4 years ago.
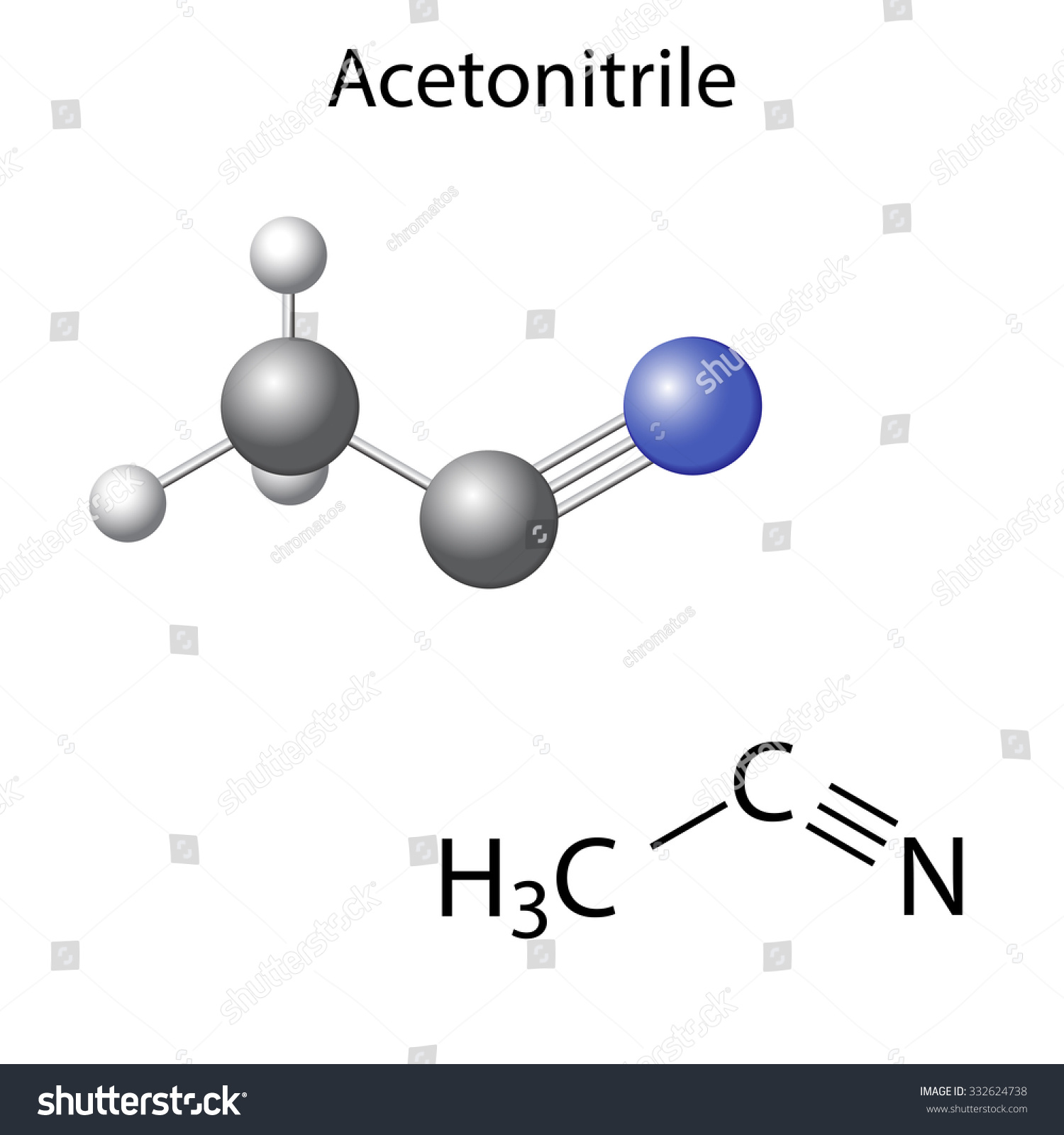
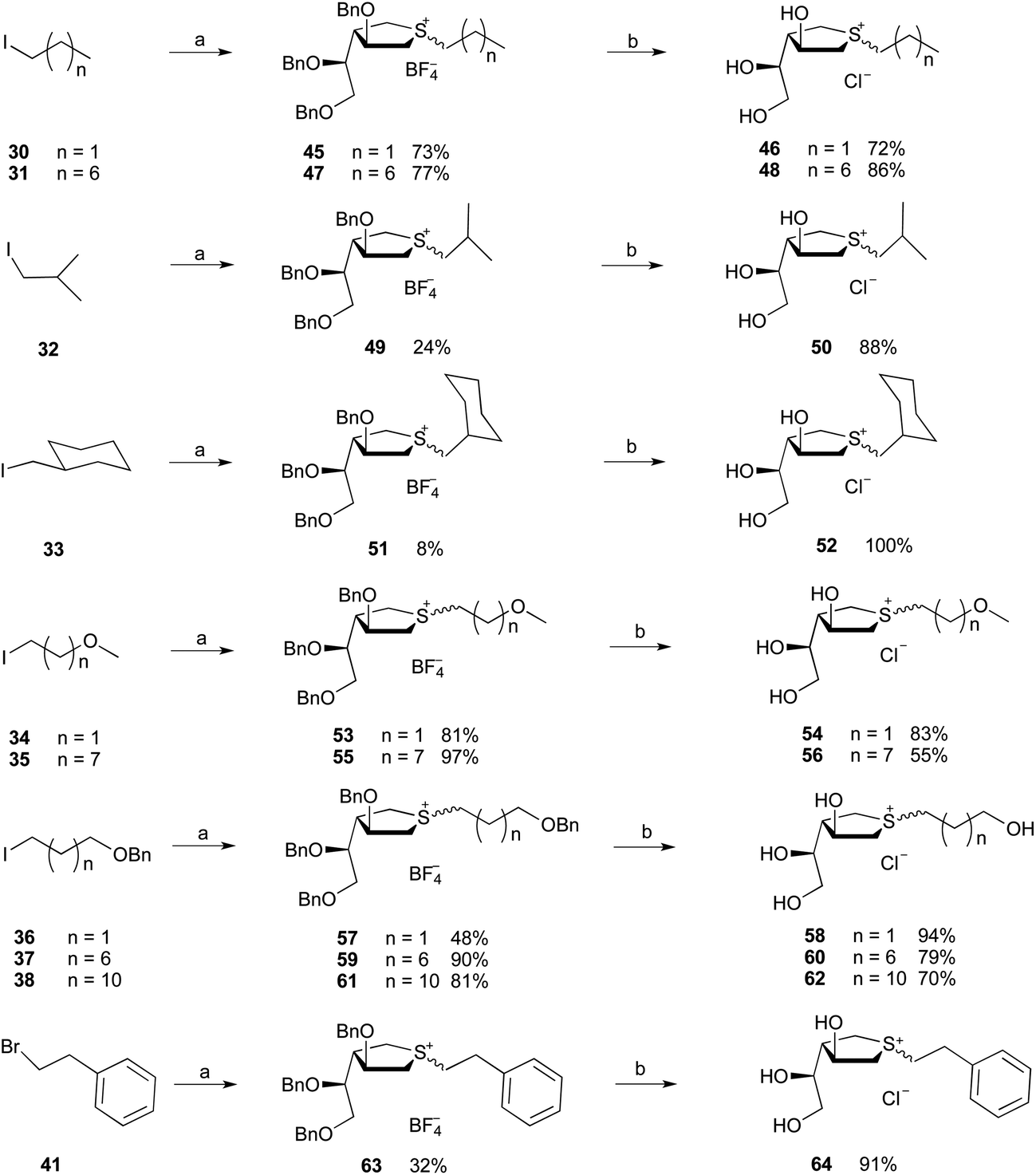
Practice Exercise p Sigma and Pi Bonds. Use the Lewis structure of acetonitrile to determine bond angles, hybridization, and the number of sigma and pi bonds present.
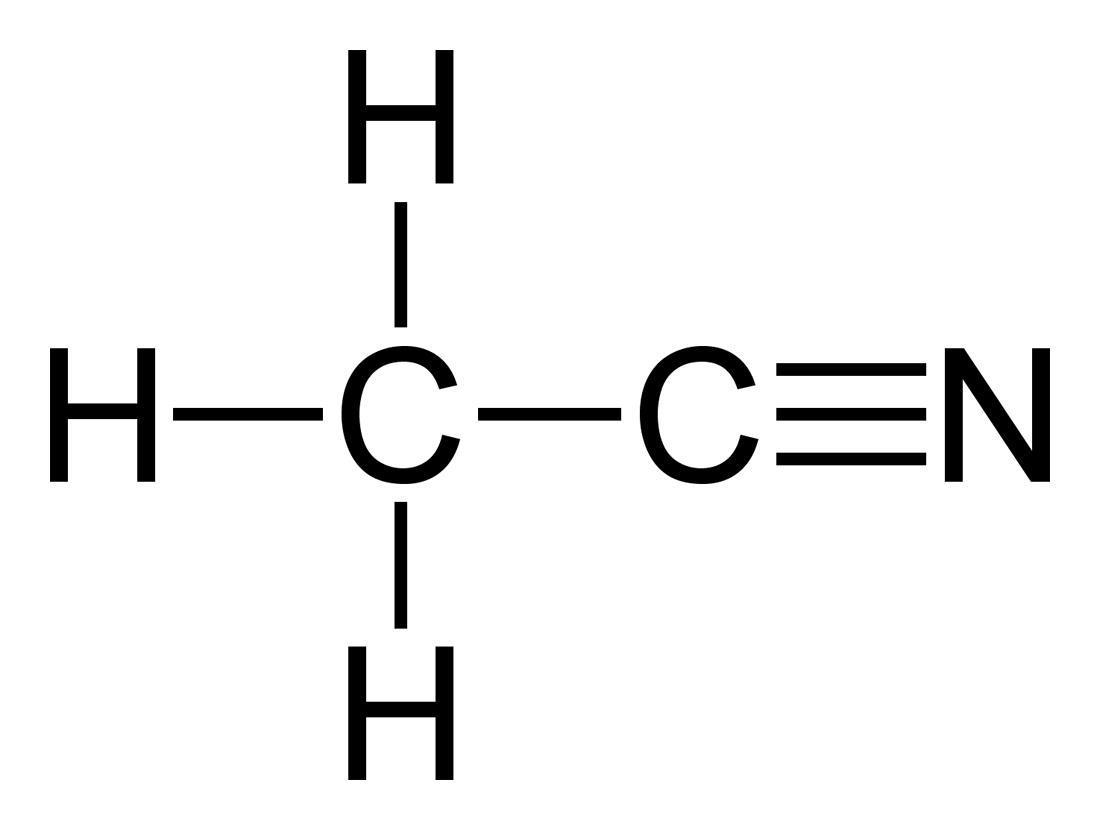
Acetonitrile is the chemical compound with the formula CH 3 CN. This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic).
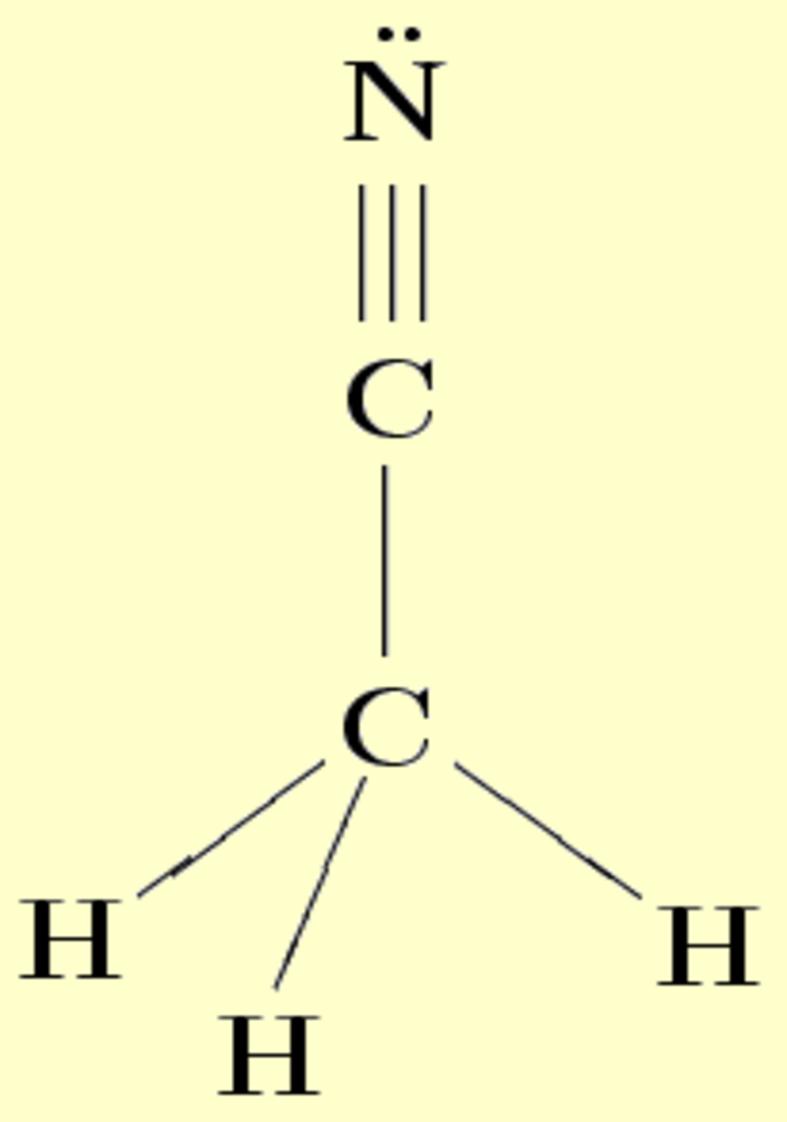
Transcript: Let’s do the CH3CN Lewis structure. For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen (we have 3 Hydrogens) plus 4 for the other Carbon and then 5 for that Nitrogen, giving us a total of 16 valence electrons.
Structure, properties, spectra, suppliers and links for: Acetonitrile, Ethanenitrile, , MeCN, NCMe, CH3CN.ShowMe – Lewis structure of CH3CNAcetonitrile – Wikipedia