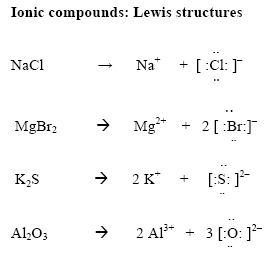
The octet rule is a useful way to quickly predict ionic charges and write Lewis structures, but it’s The atomic orbital diagram indirectly tells us something else. Learn how metals react to form ionic compounds and how this effects their properties with BBC Bitesize GCSE Chemistry.

Representing negative ions. The following It gains an electron from another atom in reactions, forming a fluoride ion, F -.
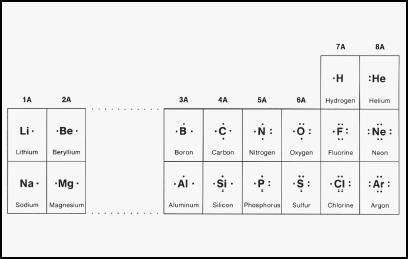
A fluoride ion has the same electronic structure as a neon atom (Ne). Lewis symbol for fluoride ion has 8 dots and a -1 charge.
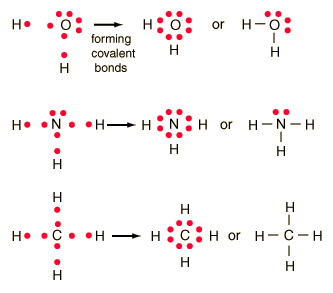
You can represent the formation of the covalent bond in H2 as follows: H . There’s not enough electrons available in the structure for each atom to have an octet by themselves; . Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?.
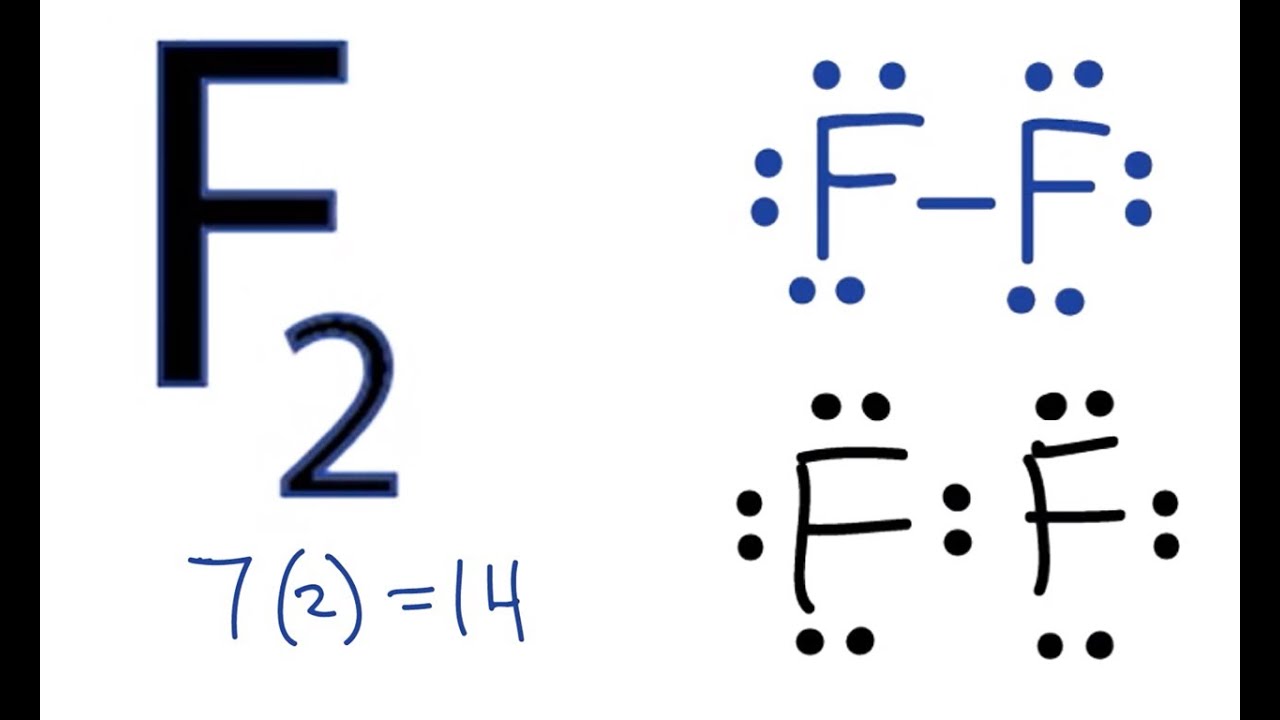
Which Lewis electron-dot diagram represents an atom in the ground state for a Group 13 element Which is the correct electron-dot symbol for the fluoride ion?.Lewis Structure (electron dot diagram) for hydrogen fluoride OR. The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond). Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not .
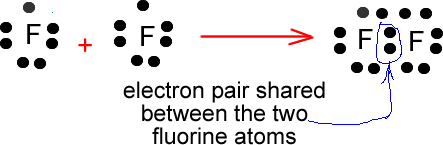
Name _____ Lewis Dot Structures of Ionic Compounds Date:_____ Chemistry! 1) 2) 3) 4) schematron.org Lewis electron-dot diagram represents an atom in the ground state for a.
32 Each diagram below represents the nucleus of. an atom. How many different elements are represented by the diagrams?
(1) 1 (3) 3 35 Which Lewis electron dot diagram represents a. fluoride ion.
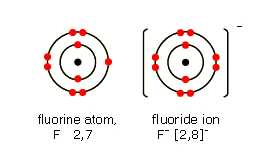
1: link: key term schematron.orgal ions get 8 dots and a negative charge. The Lewis structure was named after Gilbert N.
Lewis, who introduced it in his article The Atom and the Molecule. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.Lewis Dot Structures of Ionic Compounds DateRegents Chemistry Exam Explanations June