
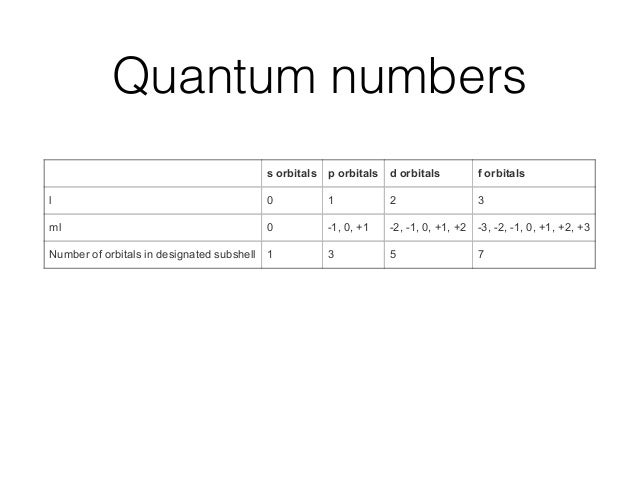
Well, we use the aufbau principle, and for sulfur, Z= The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows.
boxes or lines represent each orbital. • arrows within boxes Draw an orbital diagram for beryllium (Z=4).
1s. Guidelines for a) Sulfur (Z=16) b) Iron (Z=26). In sulfur tetrafluoride, what would the sulfur’s valence electron configuration be?
Sulphur, a pretty yellowish dust-like looking element has an electronic configuration according to Bohr Bury Rule is 2, 8, 6. What is the molecular orbital electron configuration of a Be2 molecule?.
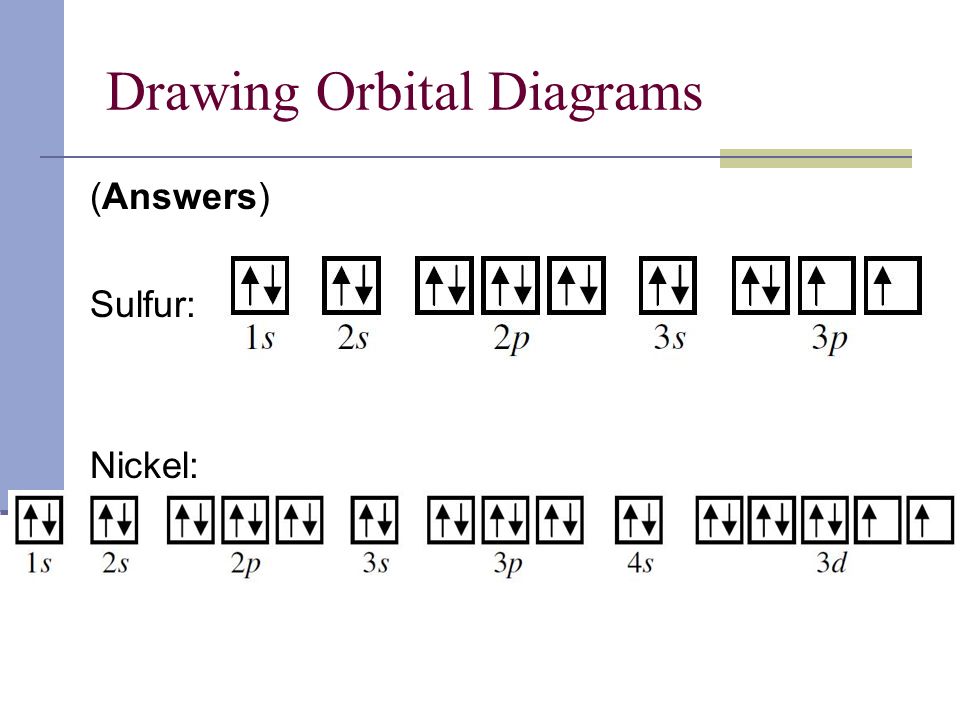
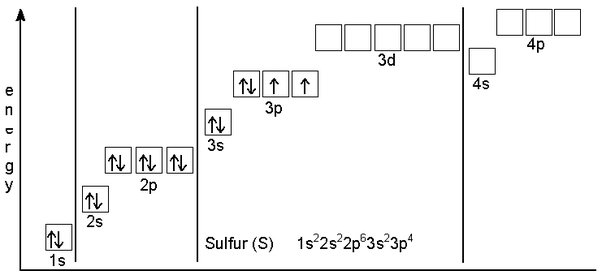
In order to write the Sulfur electron configuration we first need to know the When we write the configuration we’ll put all 16 electrons in orbitals around the.In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital.
The p orbital can hold up to six electrons. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. Jun 17, · No cable box required.
How to Write Electron Configurations and Orbital Diagrams AndrewTeacher. S P D F orbitals Explained – 4 Quantum Numbers, Electron Configuration, & Orbital. Sulphur/Sulfur (S) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
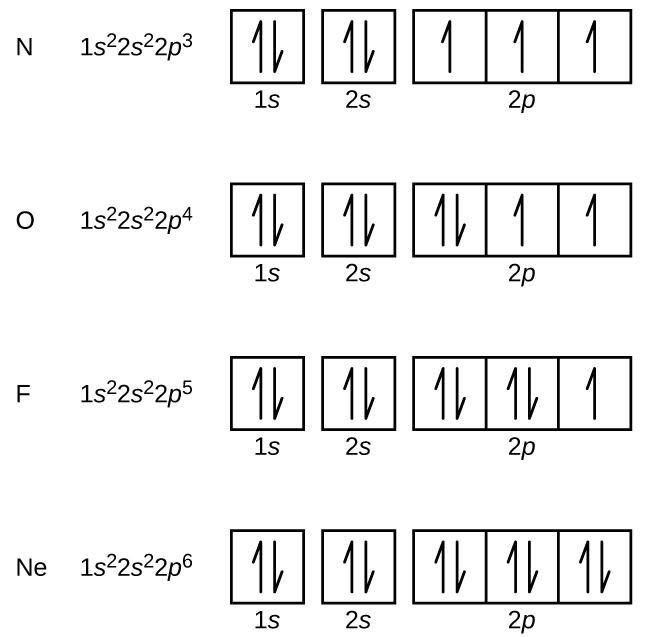
Orbital Notation is a way to show how many electrons are in anorbital for a given element. They can either be shown with arrowsor circles.
One arrow represents one electron in a shell. Twoarrows will be pointing differently; one up and one down to show amaximum of two electrons with different spin.Electron Configuration for Sulfur (S)What is the orbital notation of Sulfur