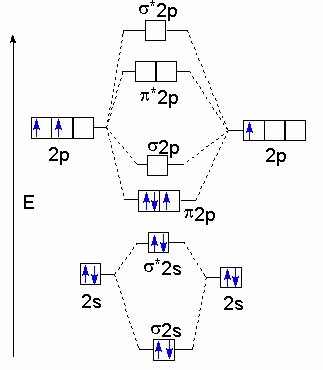
Molecular orbital theory: Atomic orbitals (AO).
Chapter 9 Energy diagrams / energy levels are often used to . theory, Ne2 doesn’t exist.
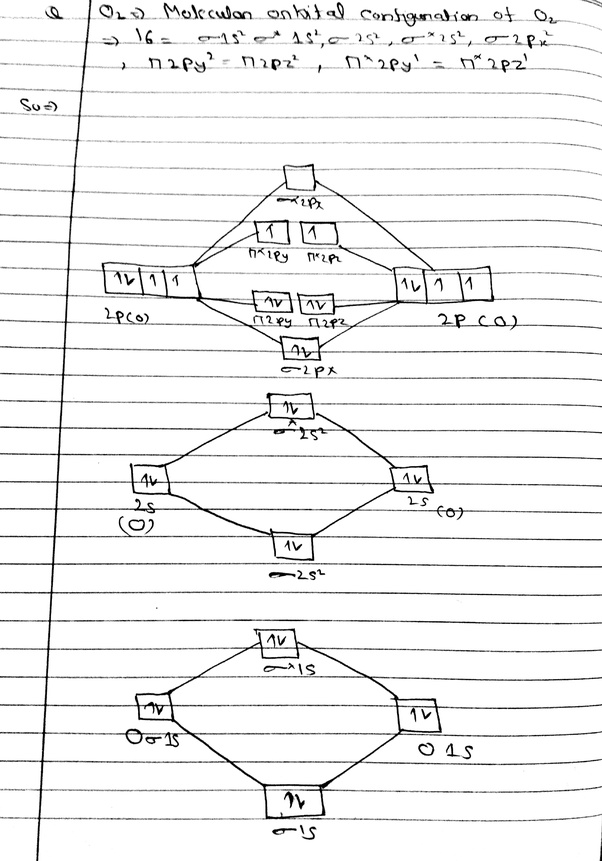
Ne2. Ignoring weak clusters held together by Van der Waals interactions, the reason that Ne2 does not form a stable covalent molecule can be.
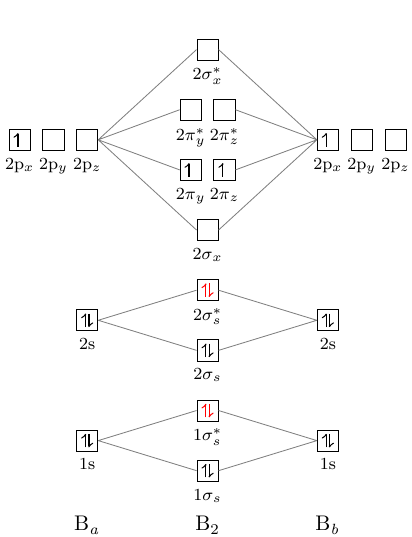
Find an answer to your question Draw and explain the molecular orbital diagram of Ne2. On the basis of molecular orbital diagram, explain.
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
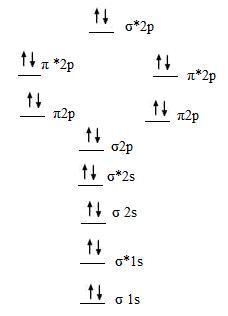
If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix? Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
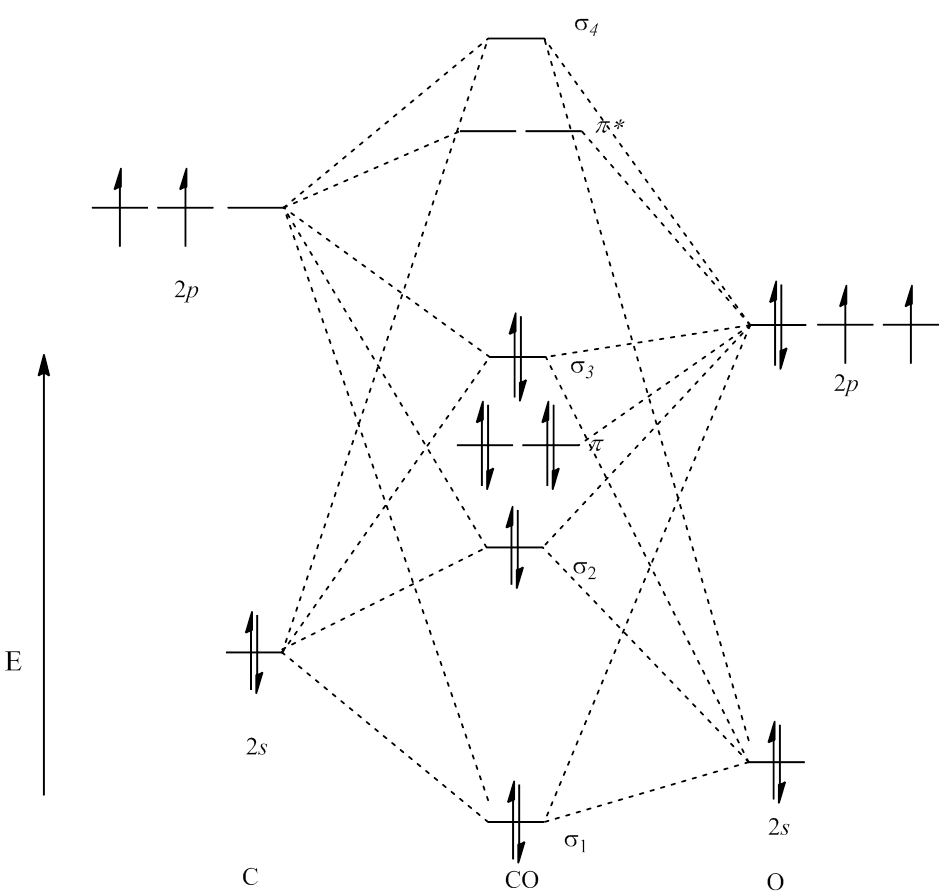
Molecular orbital diagram
The orbital correlation diagram in predicts the same thing–two electrons fill a single bonding molecular orbital. To further demonstrate the consistency of the Lewis structures with M.O.
theory, we will formalize a definition of bond order–the number of bonds between atoms in a molecule. For $\ce{N2-}$ there are 15 electrons.
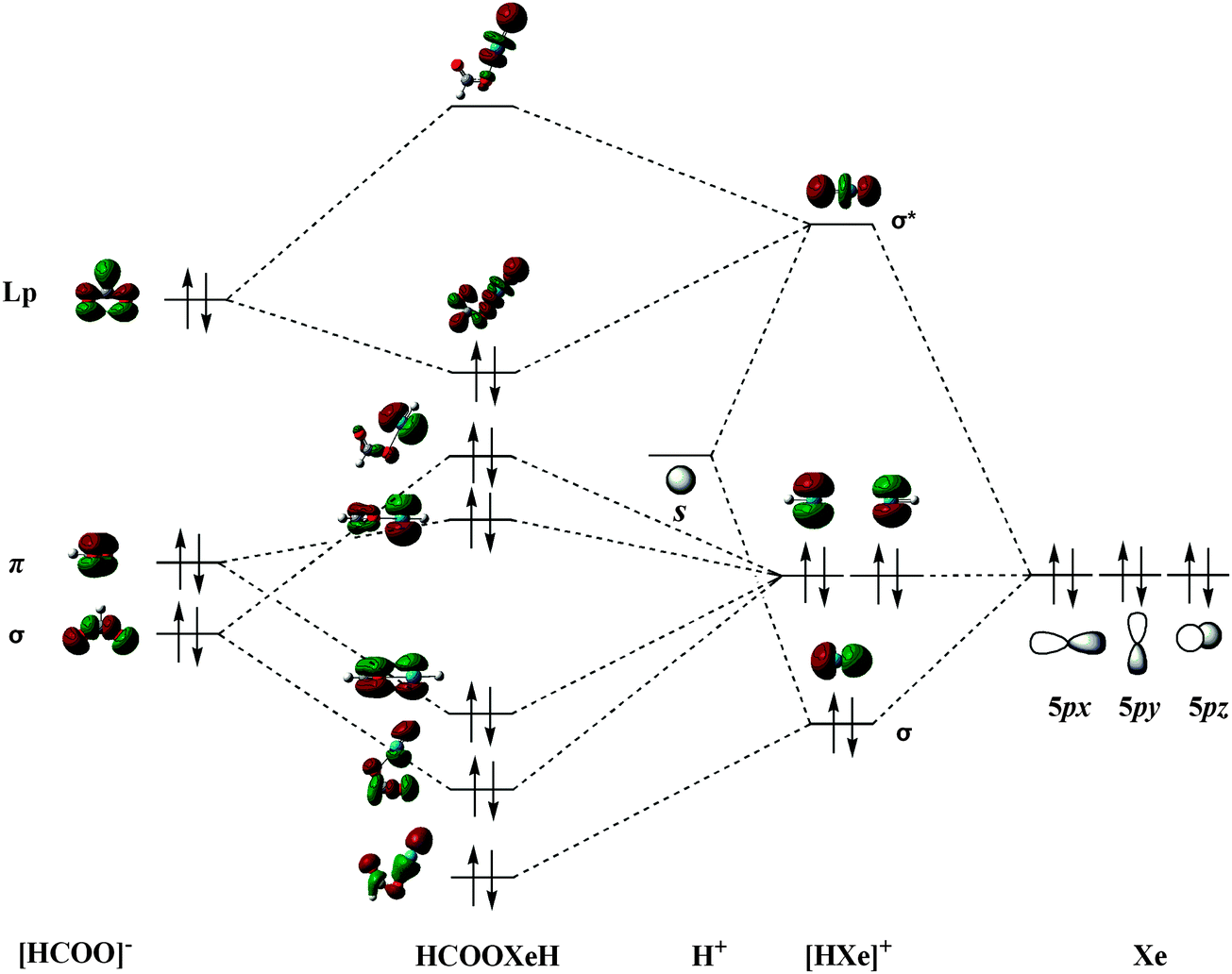
Will the MO diagram be the same as that of $\ce{N2}$ (because it is actually an ionized molecule of $\ce{N2}$) or not? Why? The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses.
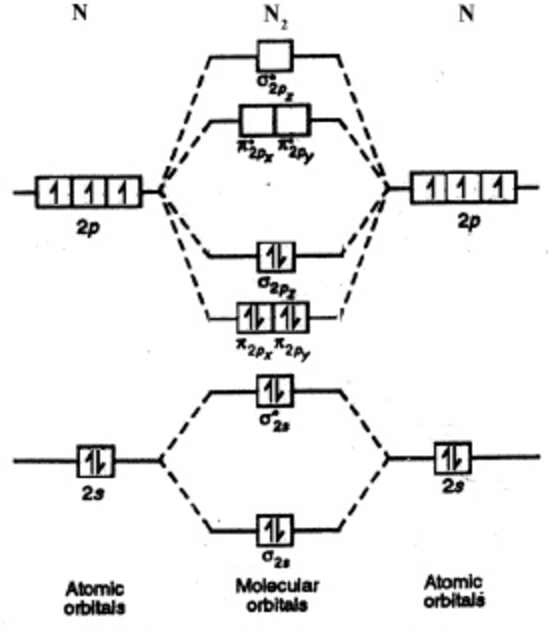
Here’s the thing, we’re going to say the one on the left deals with hydrogen, H2, to N2 on the periodic table. So hydrogen all the way to nitrogen, deals with the one on the left.
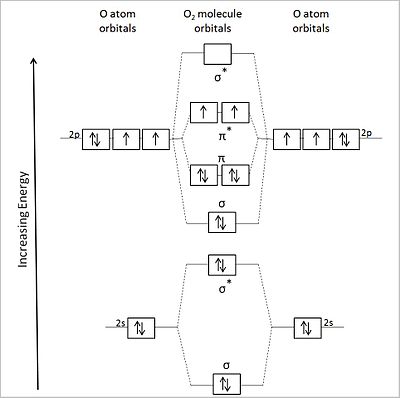
The one on the right deals with O2, F2 and Ne2. Basically, for these molecular orbital diagrams, we’re dealing with diatomic molecules.MO Energy Level Diagrams Homonuclear Molecules: O2 to Ne2Molecular orbital diagram – Wikipedia