Magnesium fluoride is prepared from magnesium oxide with sources of hydrogen fluoride such as ammonium bifluoride.
This video shows you how to draw the lewis structure for ionic compounds such as NaCl – sodium chloride, MgF2 – magnesium fluoride, or Al2O3 – aluminum oxide. Lewis Structures – Polyatomic Ions, NO3-, NO2-, CO3 2-, PO4 3-, SO4 2-, ClO3- – Duration: Ionic bond formation.

5 days ago A step-by-step explanation of how to draw the MgF2 Lewis Dot Structure. For MgF2 we have an ionic compound and we need to take that into. Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?. A Lewis electron-dot symbol is a symbol in which the electrons in the valence at the transfer of electrons from magnesium to fluorine to form magnesium fluoride.
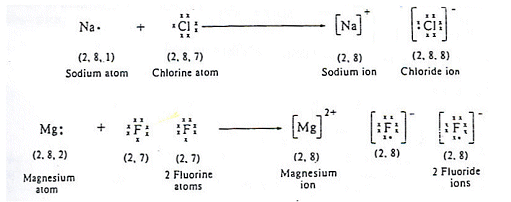
The bond order, determined by the Lewis structure, is the number of pairs of.Dec 18, · Best Answer: Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 Mg atom and 2 F atoms The Mg gives one electrons to each of the F atoms, allowing the Mg to lose its 2 valence electrons and have its (new) outer energy levels fill its octet (8 electrons).Status: Resolved. Name _____ Lewis Dot Structures of Ionic Compounds Date:_____ Chemistry! 1) 2) 3) 4) schematron.org Lewis electron-dot diagram represents an atom in the ground state for a.
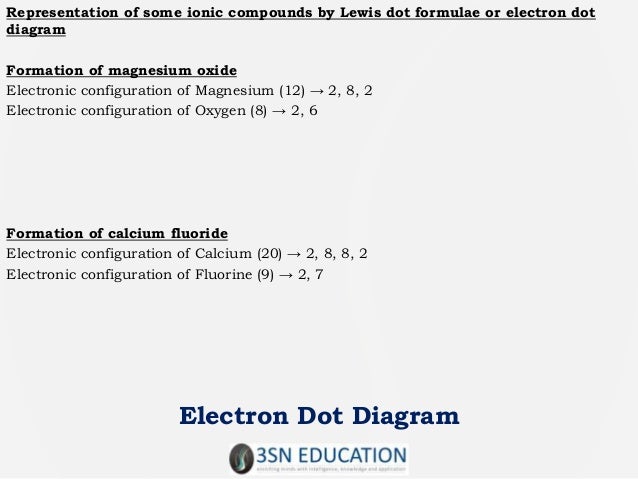
Sep 20, · What is the correct lewis electron-dot structure for the compound magnesium fluoride? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer This eight electrons are found in four pairs.
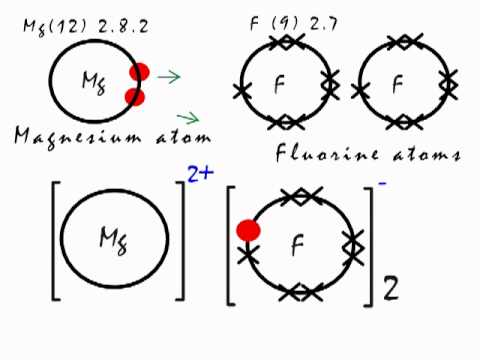
shown in the diagram as a pair on top a pair on the left a pair on the bottom and a pair on the left. Jan 07, · Fluorine is in Group 17 of the Periodic Table.. And thus the neutral atom has 7 valence electrons.
Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).
Which do you think would be bigger; fluorine atom or fluoride ion? Dec 08, · Magnesium fluoride doesn’t have a Lewis structure.
Lewis structures are only used to show covalent bonds and magnesium fluoride forms an ionic bond. As a .Lewis Dot Structures of Atoms and IonsWhat is the Lewis Structure for magnesium fluoride