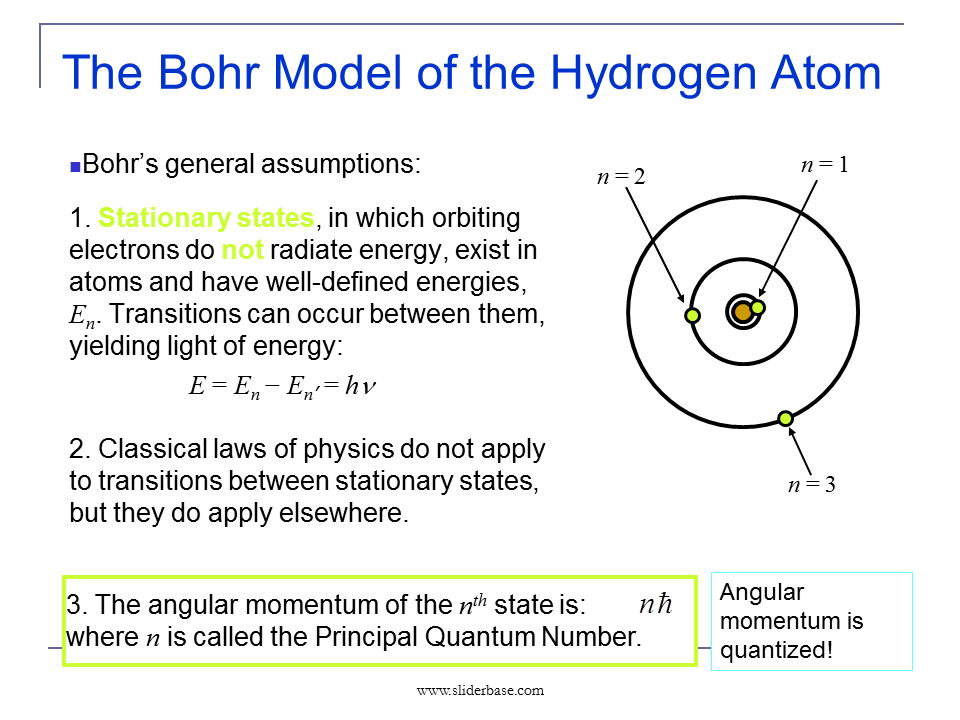
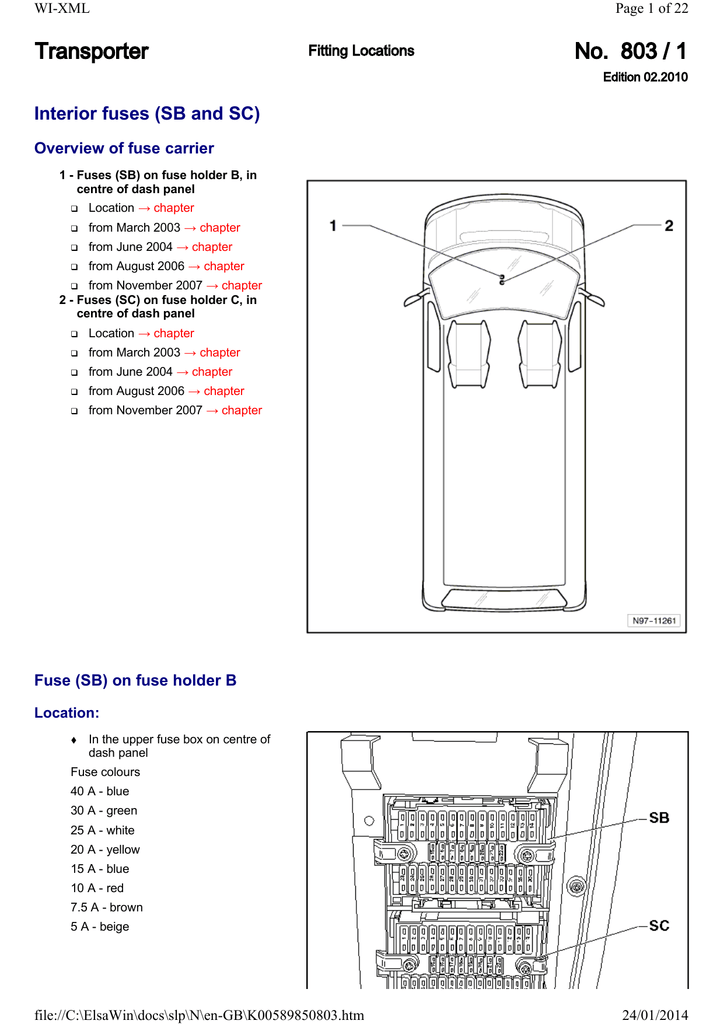
Bohr-Rutherford Diagrams & Lewis Dot Diagrams The number of dots near hydrogen and helium are the same as in the energy level chart. Why? Because the.
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like ( Hydrogen is excluded because it can hold a maximum of 2. to probe the structure of atoms under the direction of Ernest Rutherford . Once Bohr had worked out that the energy levels of hydrogen were quan- tized, i.e.
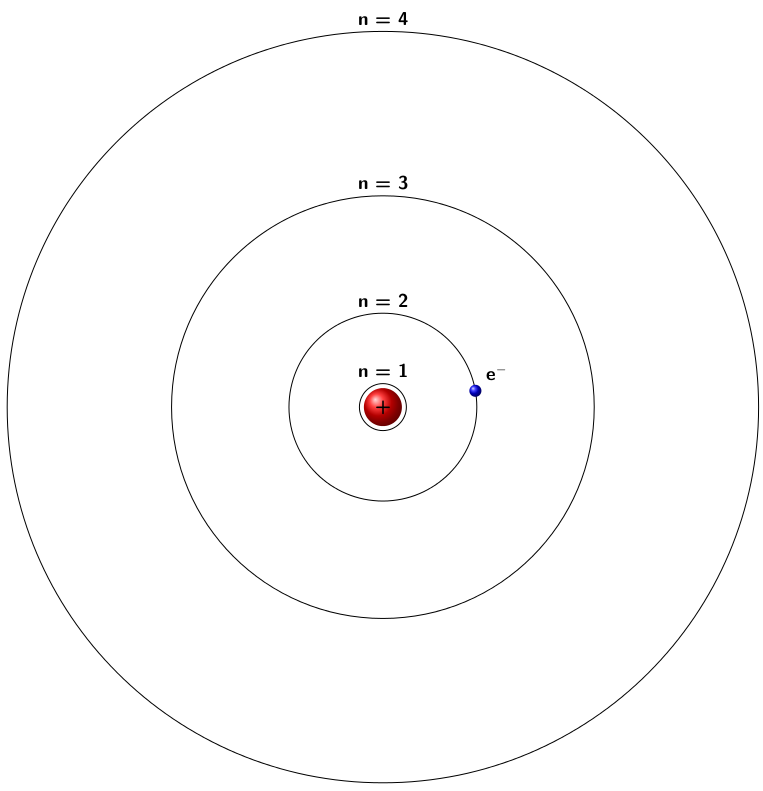
In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a. Niels Bohr introduced the atomic Hydrogen model in He described it as a positively charged nucleus, comprised of protons and neutrons.Bohr-Rutherford Diagrams We have looked at atomic models and the structure of atoms.
Today we will practice drawing those models for the elements on the periodic table. Remember that protons and neutrons are found in the nucleus of the atom and the electrons are found outside the nucleus. Bohr model of hydrogen Figure Democritus The atomic theory of matter has a long history, in some ways all the troscopy as a tool for probing atomic and molecular structure.
There are two ways in which one can observe spectral lines from an Rutherford analyzed the scattering data and developed a . draw a Bohr-Rutherford diagram for hydrogen.
draw a Bohr-Rutherford diagram for helium. draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom.
How Bohr’s model of hydrogen explains atomic emission spectra If you’re seeing this message, it means we’re having trouble loading external resources on our website. If you’re behind a web filter, please make sure that the domains *schematron.org and *schematron.org are unblocked.
Niels Bohr proposed the Bohr Model of the Atom in Because the Bohr Model is a modification of the earlier Rutherford Model, some people call Bohr’s Model the Rutherford-Bohr Model. The modern model of the atom is based on quantum mechanics.Bohr model – WikipediaBohr-Rutherford Diagrams & Lewis Dot Diagrams – Eve Wongworakul Chemistry Unit