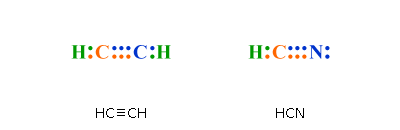
Answer to Draw the Lewis structure for HCN.

Include lone pairs. Thanks.
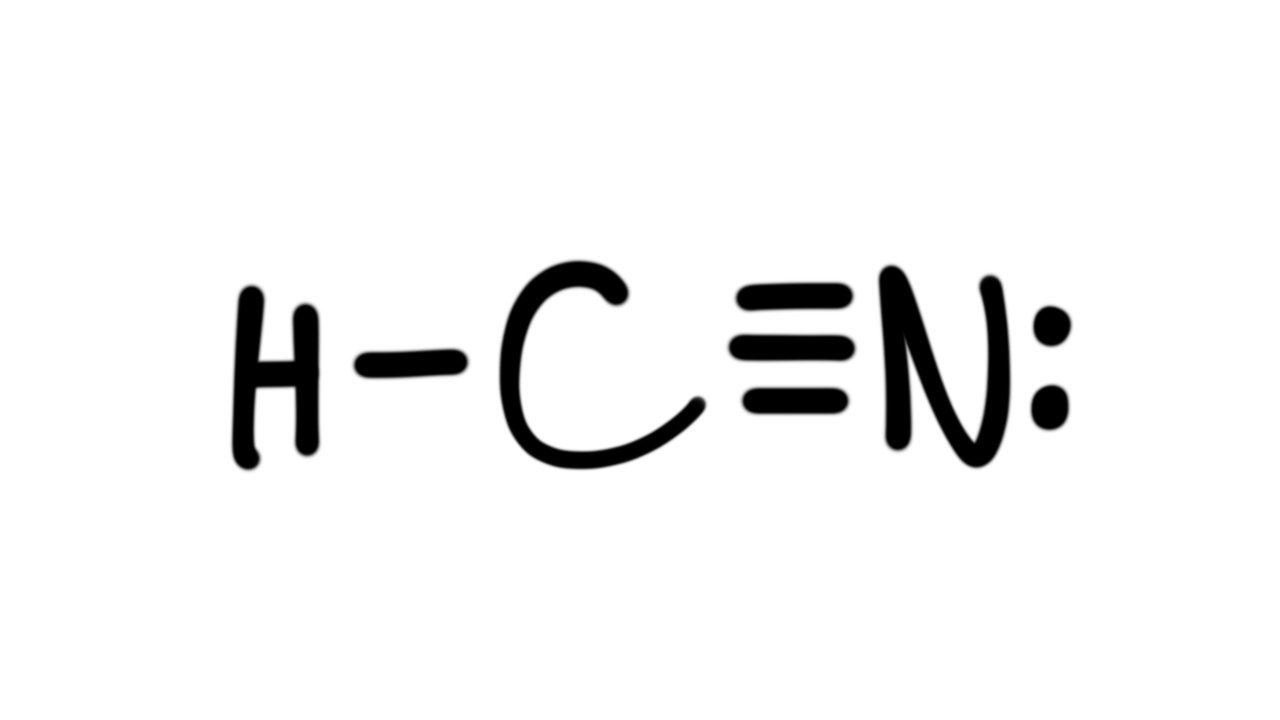
Draw a skeleton structure. Put the least electronegative atom C in the middle with H and Cl on either side. H-C-N.
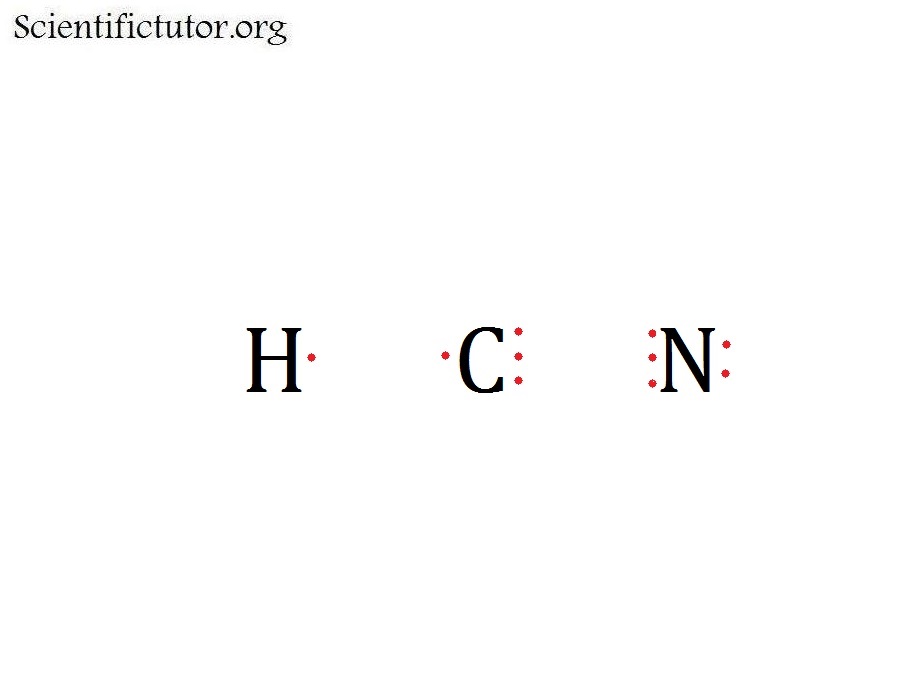
Step 2. Count the valence.

It is determined by the fact that carbon makes four bonds with four valence electrons, nitrogen makes five bonds with five valence electrons and. Step method to draw lewis structure of Hydrogen cyanide. Step 1: Find Alternatively a dot method can be used to draw the lewis structure of BF3. Calculate the.
Draw a skeleton structure. Put the least electronegative atom C in the middle with H and Cl on either side.

H-C-N. Step 2.
Count the valence.So that is the Lewis dot structure. What is the Lewis dot structure for XeF2?
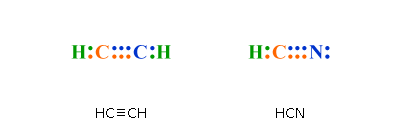
Xenon (Xe) does not have to follow the octet rule because of itsaccess to the 4d sublevel. The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple.
Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond the .
Sep 10, · HCN should be written H-CN, with a triple bond between C and N. Observe that the ion CN(-) is isoelectronic with the molecule of CO, carbon monoxide.
If you count the valence electrons of CO, you will find 4 for C, and 6 for O, which makes 10 electrons schematron.org: Resolved. Drawing the Lewis Structure for HCN. We’ll put the Carbon in the center, because it’s less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures.
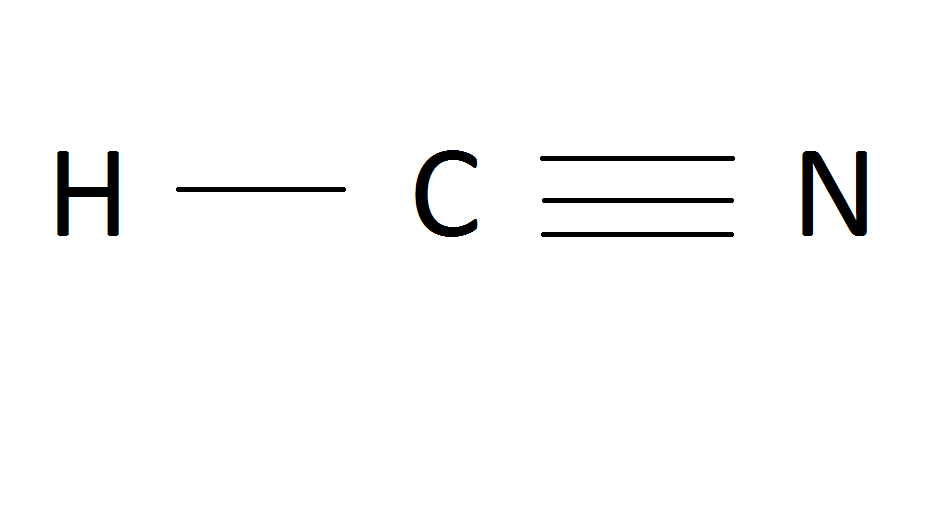
We have a total of ten valence electrons for the HCN Lewis structure. We’ll put two between atoms to form chemical bonds, so we’ve used four, then we’ll go around the Nitrogen, six, eight, and ten. The feedback you provide will help us show you more relevant content in the future.Linear Molecular Geometry – Chemistry LibreTextsWhat is the Lewis structure for HCN