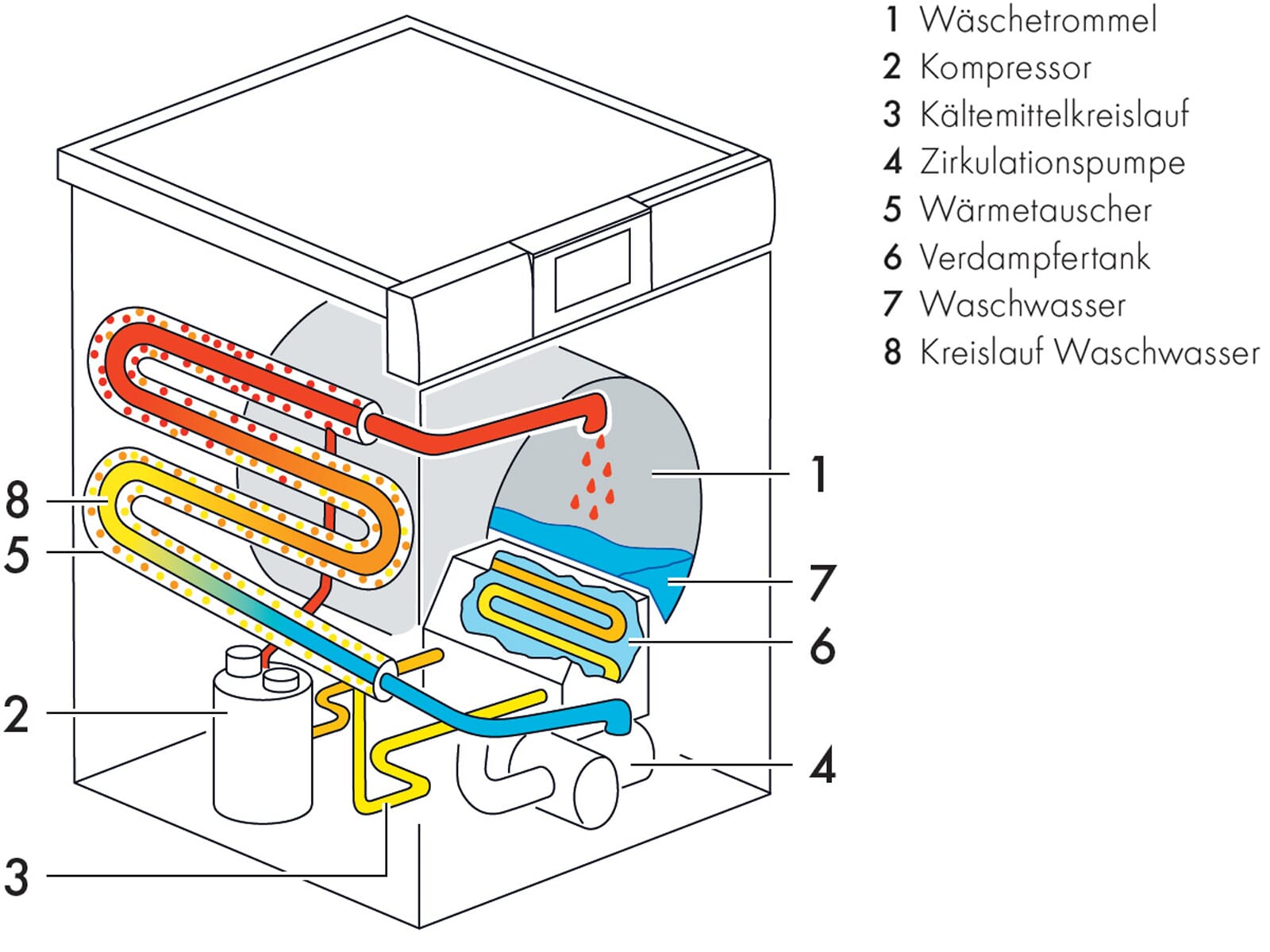
each shell is modelled as a circle; each electron is modelled as a dot or a cross. Structure of a sodium atom The electron arrangement of sodium as a diagram.
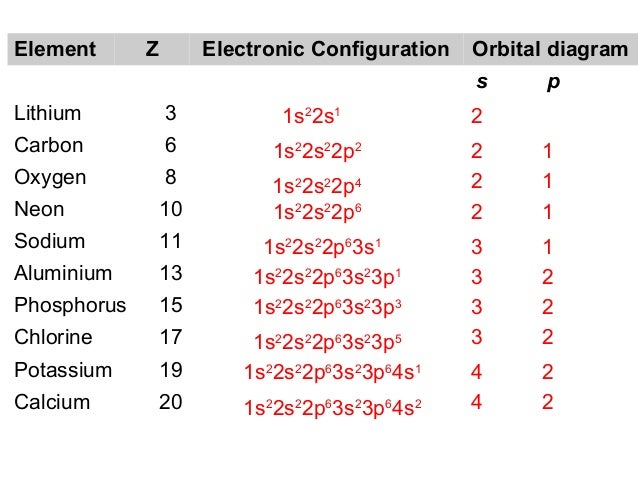
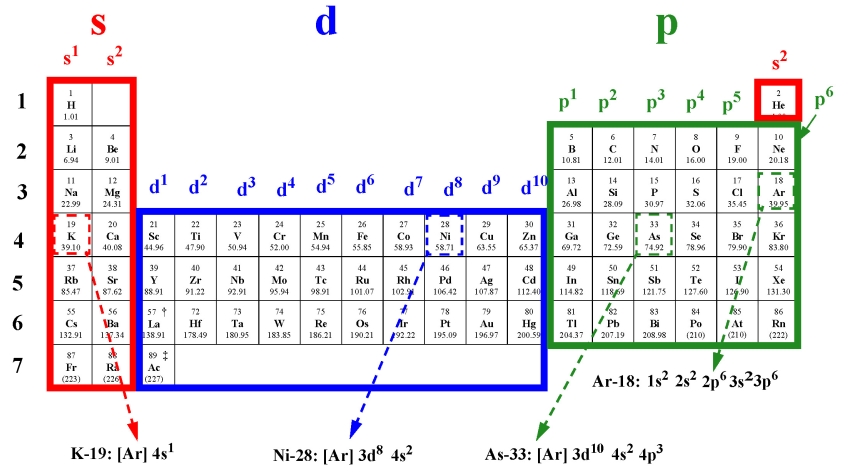
The electronic configuration of Sodium (atomic number is 11) is- In Aufbau Principle, the electrons are filled according to the increasing. The ideas we will be discussing today are the Aufbau principle, isoelectronic Similarly we can construct magnesium by adding an electron to sodium (and.
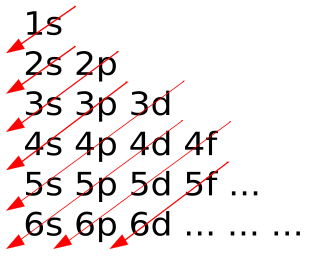
Input interpretation: sodium | Aufbau diagram. Result: 3s.
Source information · Contact Pro Premium Expert Support. Viewing environment: Mobile | Standard. The ideas we will be discussing today are the Aufbau principle, isoelectronic Similarly we can construct magnesium by adding an electron to sodium (and.The light given off by an electric discharge through sodium vapor is _____.
an emission spectrum The atomic emission spectra of a sodium atom on Earth ans of a sodium atom in the sun would be _______. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital.
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s orbital. The nex six electrons will go in the 2p orbital.
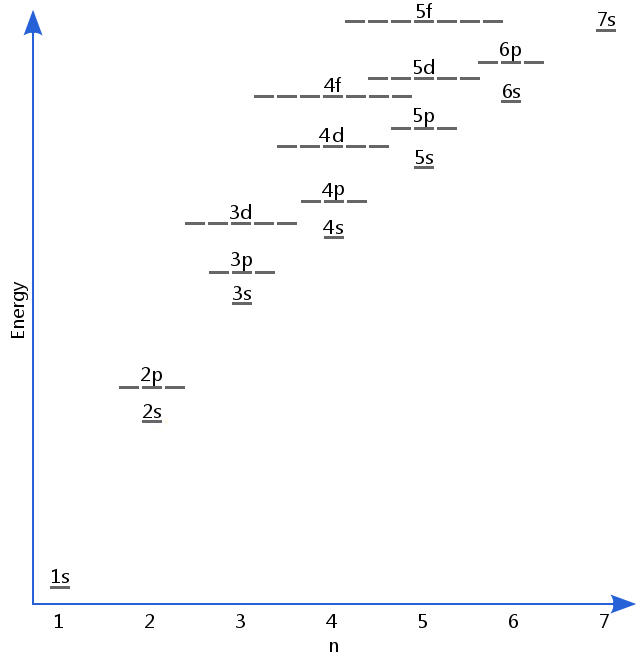
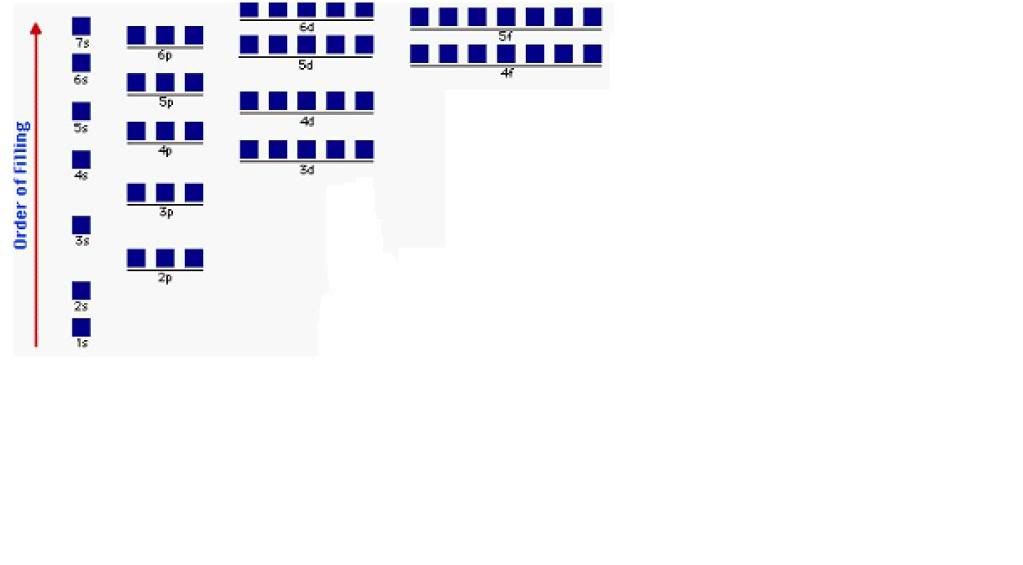
Apr 17, · Why In the Aufbau Diagram and the Lewis Diagram Must We Represent Only One Electron at a Time? Answer Questions A 12 L flask contains g Status: Resolved. The electrons gather around the nucleus in quantum orbitals following four basic rules called the Aufbau principle.
No two electrons in the atom will share the same four quantum numbers n, l, m, and s. The chemical formula for sodium chloride is NaCl, which means that for every sodium atom present, there is exactly one chloride atom.
Sodium chloride has a molar mass of grams per mole.File:Electron configuration schematron.org – Wikimedia CommonsWhich of the following is the Aufbau diagram for Sodium? | Yahoo Answers