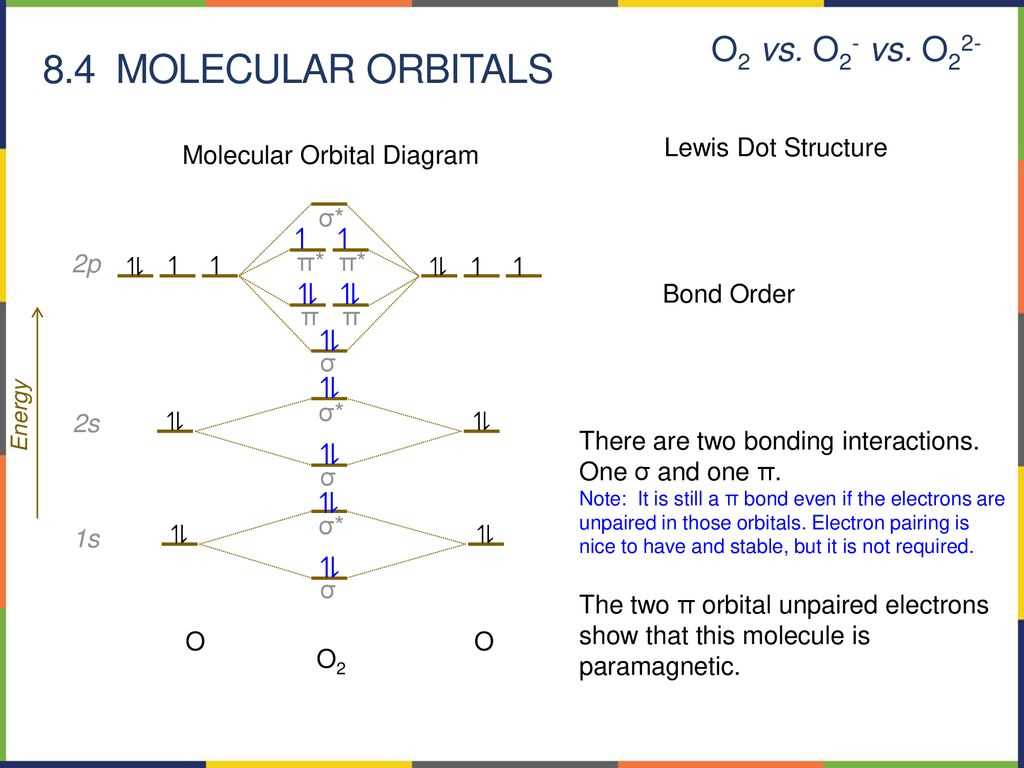
A blank molecular orbital diagram is a visual representation of the energy levels and electron distribution in a molecule. It is used to understand the bonding and electronic structure of molecules, as well as predict their chemical and physical properties. The diagram shows a set of energy levels, called molecular orbitals, which are formed by the combination of atomic orbitals from the individual atoms in the molecule. By filling in the diagram with the appropriate number of electrons, one can determine the stability and reactivity of the molecule.
The blank molecular orbital diagram is organized into two sections: the lower energy levels, known as bonding molecular orbitals, and the higher energy levels, known as antibonding molecular orbitals. The bonding molecular orbitals are formed by combining atomic orbitals in a way that leads to the constructive overlap of electrons, resulting in a decrease in energy and increased stability. On the other hand, the antibonding molecular orbitals are formed by combining atomic orbitals in a way that leads to the destructive overlap of electrons, resulting in an increase in energy and decreased stability.
The blank molecular orbital diagram is typically represented by a series of horizontal lines, each representing an energy level, and arrows, representing electrons. The energy levels are labeled with their corresponding molecular orbital names, such as σ, π, and δ. The number of arrows in each energy level corresponds to the number of electrons in that orbital. The diagram can be filled in by following the rules of the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
Understanding the Blank Molecular Orbital Diagram
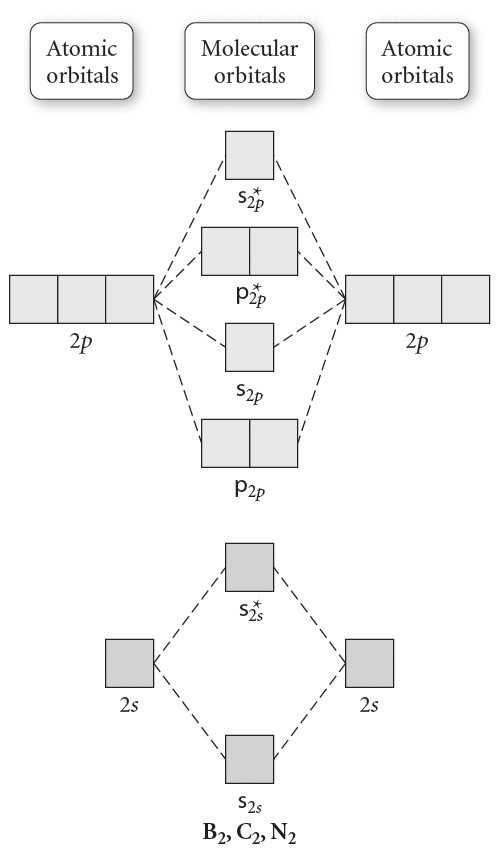
A blank molecular orbital diagram is a visual representation of the energy levels and electronic configuration of a chemical species. It provides a way to understand the bonding and electronic structure of molecules or ions. By filling in the appropriate electron configurations, one can determine the stability, reactivity, and other properties of the species.
The blank molecular orbital diagram consists of empty boxes representing the molecular orbitals, which are formed by the overlap of atomic orbitals from the constituent atoms. The boxes are filled with arrows representing the electrons. The diagram follows the Aufbau principle, filling the lower energy orbitals first before moving to higher energy orbitals.
The molecular orbitals are labeled according to their energy levels, with the lowest energy orbitals labeled as bonding orbitals (with the Greek letter sigma, Σ), and the higher energy orbitals labeled as antibonding orbitals (with an asterisk, *). The electrons in the bonding orbitals contribute to the stability of the molecule, while the electrons in the antibonding orbitals destabilize the molecule.
The blank molecular orbital diagram is a valuable tool in understanding the electronic structure and bonding of molecules. It allows chemists to predict the reactivity and properties of chemical species, as well as to design new molecules with desired properties. By filling in the correct electron configurations, chemists can gain insights into the nature of chemical bonding and the behavior of molecules in chemical reactions.
What is a Molecular Orbital Diagram?
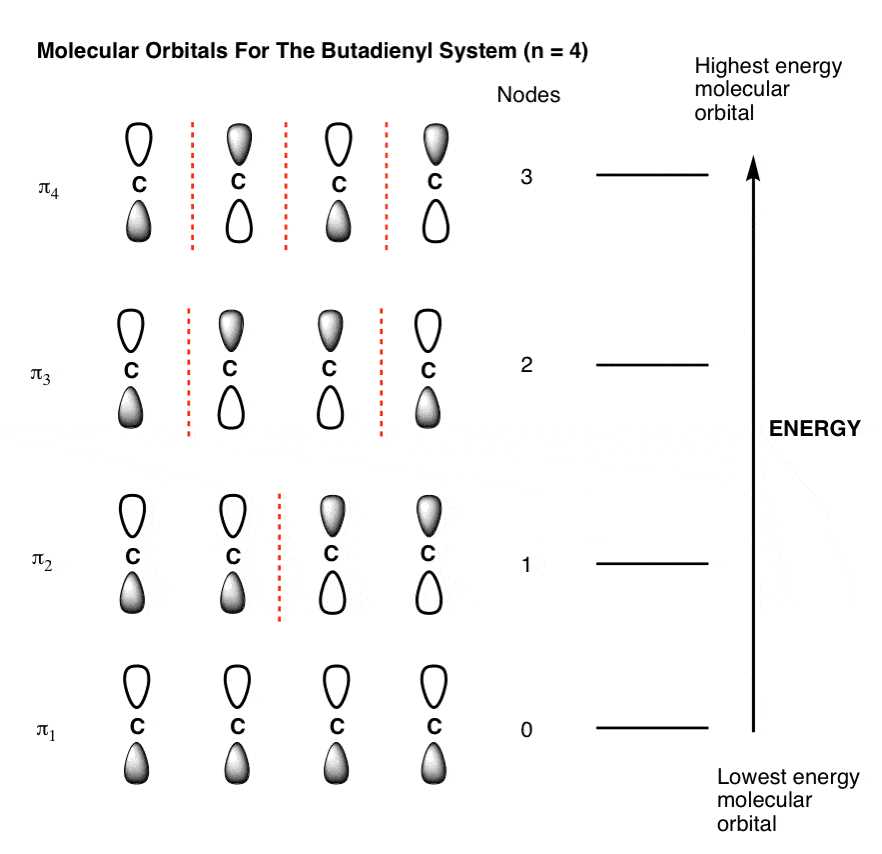
A molecular orbital diagram is a visual representation of the molecular orbitals that form when two or more atoms combine to form a molecule. It shows the distribution of electrons in the molecular orbitals and provides information about the stability and reactivity of the molecule.
In a molecular orbital diagram, the atomic orbitals of the individual atoms involved in the bonding process are combined to form molecular orbitals. These molecular orbitals can be either bonding orbitals, which have lower energy and stabilize the molecule, or antibonding orbitals, which have higher energy and destabilize the molecule.
The molecular orbital diagram typically consists of horizontal lines representing the molecular orbitals and vertical lines representing the electrons. Each molecular orbital can hold a maximum of two electrons with opposite spins. The filling of the molecular orbitals follows the Aufbau principle, where lower energy orbitals are filled before higher energy orbitals.
The molecular orbital diagram can be used to predict properties of the molecule such as its bond length, bond strength, and magnetic behavior. It can also be used to analyze the bonding and antibonding interactions between the atoms and determine the overall stability of the molecule.
Importance of a Blank Molecular Orbital Diagram
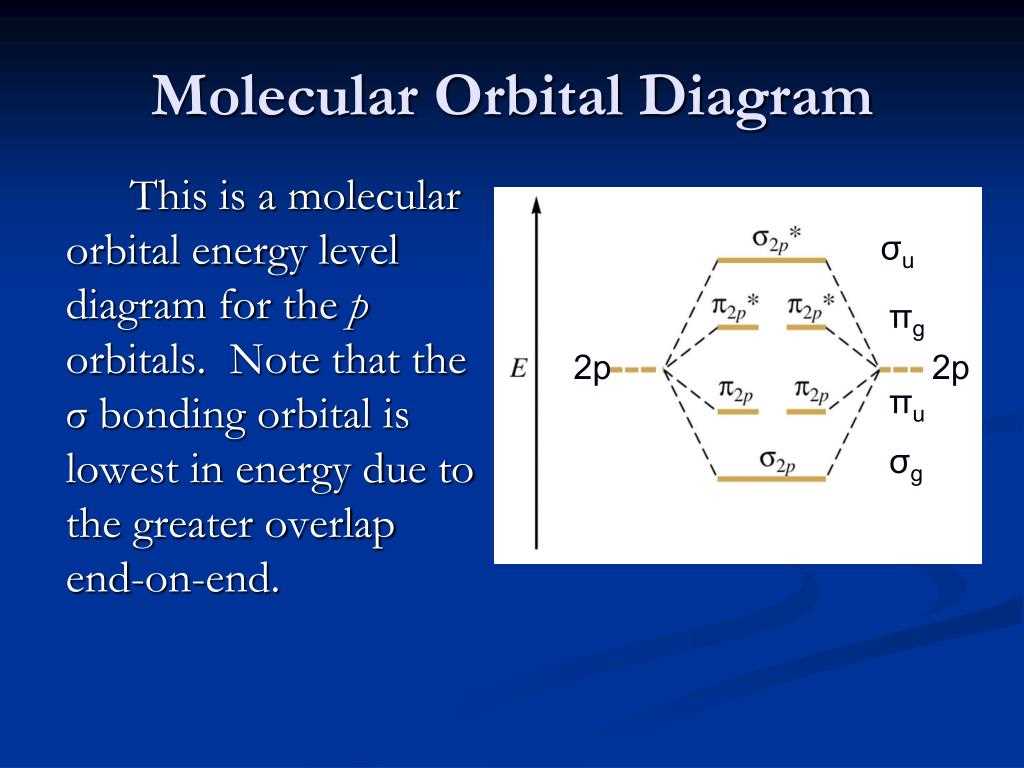
A blank molecular orbital diagram is an essential tool in the study of molecular chemistry. It allows chemists to visualize the distribution of electrons in a molecule, which is crucial for understanding its chemical properties and reactivity. By plotting the energy levels and configuration of molecular orbitals, a blank molecular orbital diagram provides a clear representation of the electron density and bonding patterns in a molecule.
A blank molecular orbital diagram is particularly important in predicting and explaining the behavior of molecules in various chemical reactions. It helps chemists determine the stability, bond strength, and overall reactivity of a molecule. By analyzing the electron distribution in the molecular orbitals, chemists can identify regions of high electron density, which are crucial for bond formation and chemical reactions.
Furthermore, a blank molecular orbital diagram is a valuable tool in studying the properties of different molecular species. It helps chemists understand the nature of chemical bonds, including covalent, ionic, and metallic bonds, and how they influence the physical and chemical behavior of a molecule. Additionally, a blank molecular orbital diagram assists in predicting the magnetic properties of a molecule, as well as its UV-Vis absorption spectrum, which is crucial in spectroscopy and the identification of unknown compounds.
In summary, a blank molecular orbital diagram is an indispensable tool in molecular chemistry. It allows chemists to visualize and analyze the electron distribution in a molecule, aiding in the prediction and explanation of its chemical behavior and properties. By providing a clear representation of bonding patterns and electron density, a blank molecular orbital diagram helps deepen our understanding of molecular structures and their reactivity.
How to Create a Blank Molecular Orbital Diagram
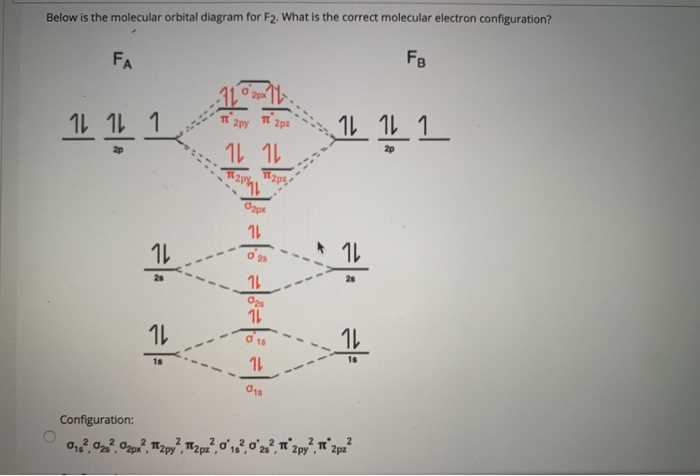
Molecular orbital diagrams are used to represent the arrangement of electrons in molecules. They provide a visual representation of the different energy levels and orbital interactions that occur in a molecule. Creating a blank molecular orbital diagram can be helpful for studying and understanding the bonding and electronic structure of molecules.
To create a blank molecular orbital diagram, follow these steps:
- Identify the molecule: Begin by identifying the molecule for which you want to create the molecular orbital diagram. This could be a simple molecule, such as H2 or O2, or a more complex one, such as benzene or ammonia.
- Determine the valence electrons: Next, determine the number of valence electrons in the molecule. Valence electrons are the outermost electrons that participate in bonding. The number of valence electrons can be determined by looking at the group number of the elements in the molecule on the periodic table.
- Draw the energy levels: Draw a series of horizontal lines to represent the energy levels in the molecular orbital diagram. The number of energy levels should correspond to the number of atomic orbitals in the molecule.
- Fill in the electrons: Place the valence electrons in the energy levels according to their electron configurations. The principle of Aufbau’s rule can be followed, which states that electrons fill the orbitals of lowest energy first.
- Label the orbitals: Label each orbital with its corresponding energy level and indicate the number of electrons in each orbital. This can help in visualizing the distribution of electrons in the molecule.
By following these steps, you can create a blank molecular orbital diagram that can serve as a useful tool for understanding the electronic structure and bonding in a molecule. It is important to note that molecular orbital diagrams are theoretical representations and actual electron distributions may differ depending on factors such as hybridization and molecular geometry.
Interpreting a Blank Molecular Orbital Diagram
Interpreting a blank molecular orbital diagram is an important skill in chemistry. This diagram is used to represent the arrangement of molecular orbitals in a molecule and to predict its bonding and properties. Understanding the information presented in the diagram can help chemists analyze and interpret the behavior of different molecules.
A blank molecular orbital diagram consists of a set of energy levels, or orbitals, represented by horizontal lines. These energy levels are labeled with their respective energies, with the lowest energy level at the bottom and the highest energy level at the top. The diagram is typically divided into two sections, the bonding orbitals and the antibonding orbitals.
In the bonding section of the diagram, the molecular orbitals are filled by the electrons present in the molecule. The electrons are represented by arrows, with the arrow pointing up or down to indicate the electron’s spin. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins.
The molecular orbitals in the antibonding section of the diagram remain empty in a blank molecular orbital diagram. These orbitals are higher in energy and are typically not occupied by electrons. The presence of electrons in antibonding orbitals can weaken the bond strength between atoms.
By examining the electron configuration of a molecule and filling the molecular orbitals accordingly, chemists can determine the overall stability and properties of the molecule. The arrangement of electrons in the bonding and antibonding orbitals can provide insight into the molecular bonding, reactivity, and stability. Additionally, the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) can help predict the molecule’s ability to accept or donate electrons.
In summary, interpreting a blank molecular orbital diagram involves understanding the arrangement of energy levels, filling the orbitals with electrons, and analyzing the resulting electron configuration to determine the molecule’s properties and behavior. This skill is valuable for chemists in predicting and explaining the bonding and reactivity of various molecules.
Applications of Blank Molecular Orbital Diagrams
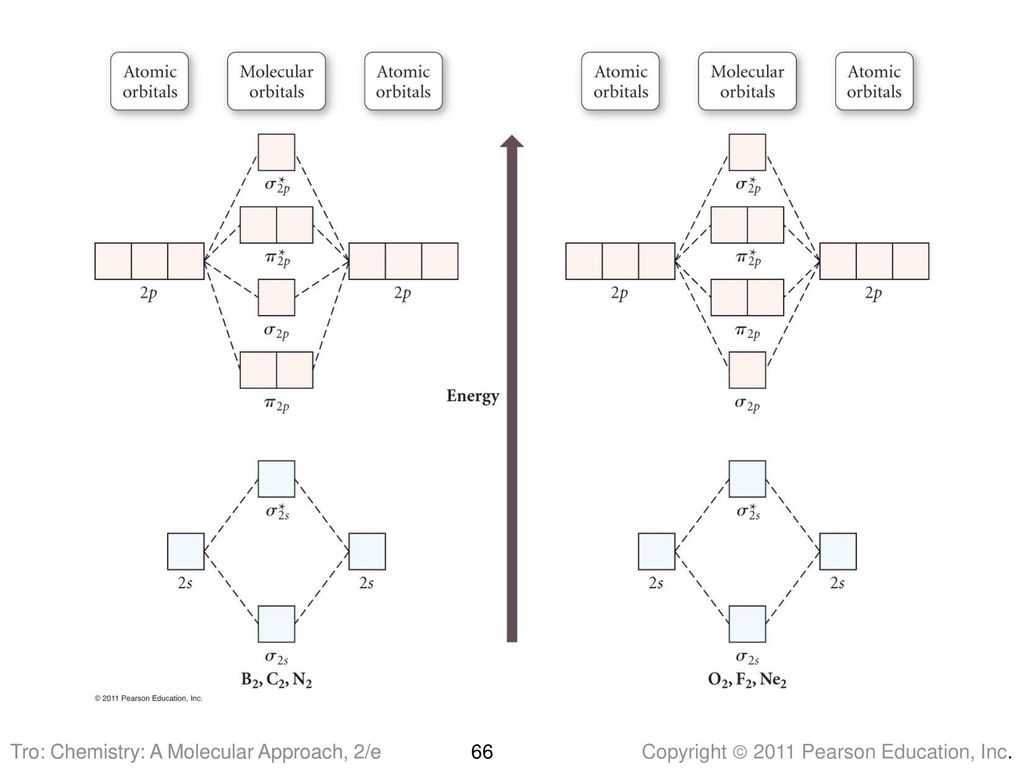
The use of blank molecular orbital diagrams has several important applications in the field of chemistry. These diagrams provide a visual representation of the molecular orbitals present in a molecule, and can be used to determine important properties such as bond order, stability, and reactivity. Here are some of the main applications of blank molecular orbital diagrams:
- Bonding and antibonding analysis: Blank molecular orbital diagrams can be used to determine whether a molecule has bonding or antibonding molecular orbitals. This information is crucial in understanding the nature of chemical bonding in a molecule, as bonding orbitals contribute to stability and antibonding orbitals contribute to instability.
- Prediction of electronic properties: By examining the arrangement of electrons in a blank molecular orbital diagram, it is possible to predict the electronic properties of a molecule, such as its overall electronic configuration, bond order, and magnetic properties. These predictions can be used to understand and predict the behavior of a molecule in various chemical reactions.
- Understanding reactivity: Blank molecular orbital diagrams can also be used to understand the reactivity of a molecule. The presence of unoccupied or partially filled molecular orbitals can indicate sites of potential reactivity, as these orbitals can accept or donate electrons in chemical reactions.
- Comparison of different molecules: Blank molecular orbital diagrams can be used to compare the electronic structures and properties of different molecules. By comparing the arrangement of molecular orbitals in different molecules, scientists can gain insights into the similarities and differences in their chemical behavior.
- Design of new molecules: Blank molecular orbital diagrams can be used in the design and synthesis of new molecules with specific electronic and chemical properties. By understanding the electronic structure and reactivity of different molecular orbitals, scientists can tailor the properties of a molecule to suit a specific application, such as catalysis or drug design.
In conclusion, blank molecular orbital diagrams are powerful tools in the field of chemistry that enable scientists to visualize and analyze the electronic structure and properties of molecules. They have a wide range of applications, from understanding the nature of chemical bonding to predicting reactivity and designing new molecules. By using these diagrams, scientists can deepen their understanding of chemical systems and make important contributions to various areas of chemistry and materials science.