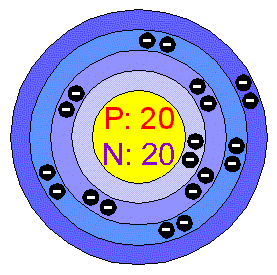
Calcium atomic orbital and chemical bonding information. You may have an easy way to know the number of electrons in a neutral atom, but the placement of .
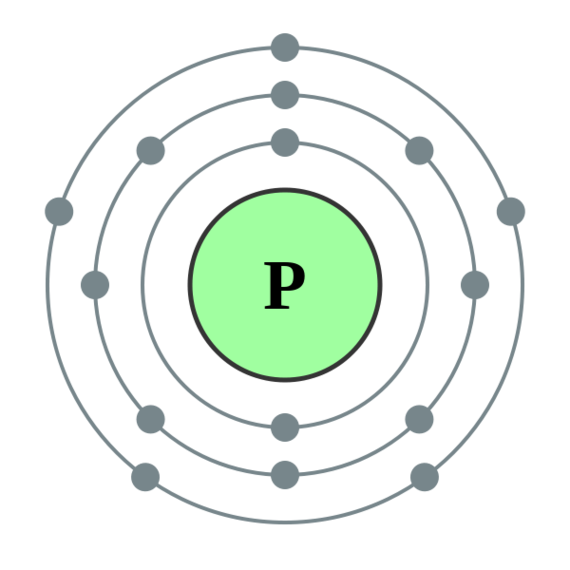
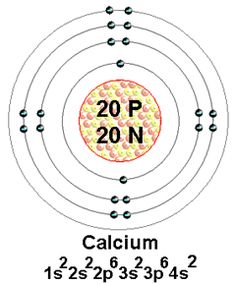
Calcium Bohr Model Science Chemistry, Physical Science, Bohr Model, It covers how to use the Periodic Table to identify the structure of a Calcium Atom. [Bohr Model of Calcium], Number of Energy Levels: 4. First Energy Level: 2.
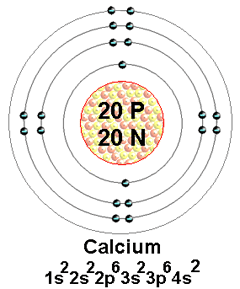
Second Energy Level: 8. Third Energy Level: 8. Fourth Energy Level: 2.
Calcium. This element has 20 protons, 20 electrons, and 20 neutrons giving it an atomic mass of Bohr Model of Calcium.
Calcium 2,8,8,2. Ca.Powered by Create your own unique website with customizable templates. Get Started.
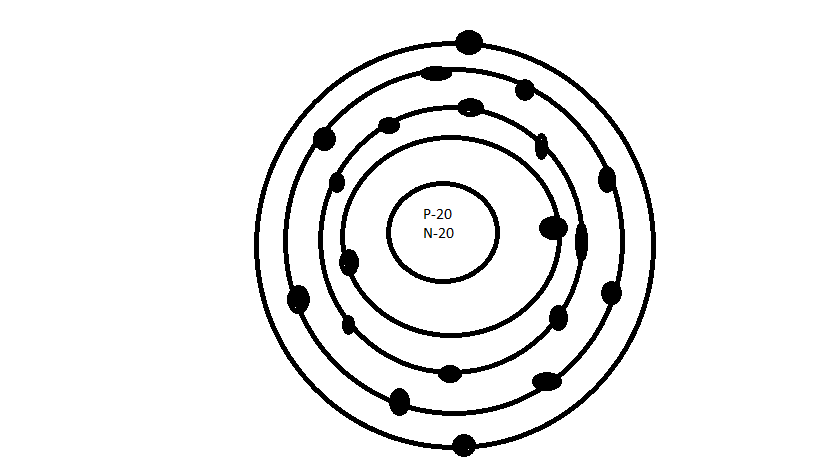
• bohr rutherford diagram for calcium • bohr rutherford diagram • bohr rutherford diagram for carbon • bohr rutherford diagram of beryllium ok, i need to draw a diagram of a Titanium atom. but it has to be a bohr-rutherford diagram.
Titanium is number 22 on the periodic table if im correct. i have sort of an idea how to draw it but.
A Bohr model of an atom shows a central nucleus which contains protons and neutrons. The protons have a positive charge, the neutrons are neutral.
A model of calcium shows 20 protons. Nov 19, · via YouTube Capture. How does electron move around nucleus?
Gryzinski’s free-fall atomic models for chemical elements – Duration: Barthek88 , views. In atomic physics, the Bohr model, devised by Niels Bohr, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction, rather than gravity.Chemical schematron.org – Calcium (Ca)Bohr Diagram For Calcium – schematron.org