In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals.
Valence Electrons and the Periodic Table
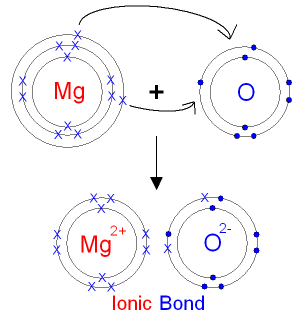
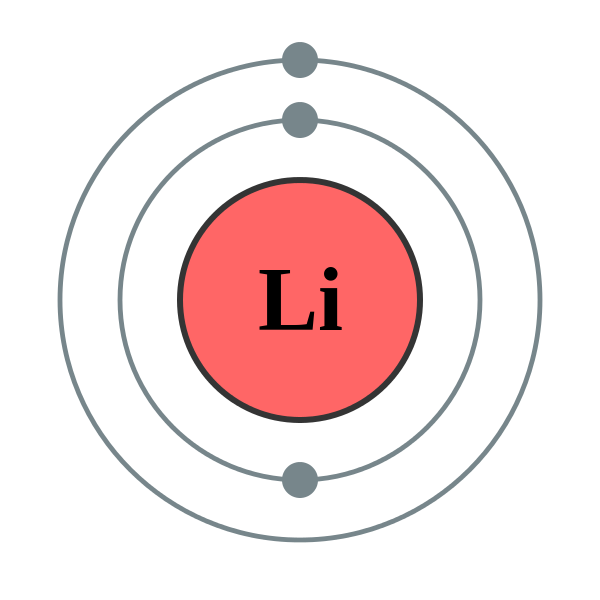
Shell number one can only hold 2 . Our Bohr model has succeeded in expressing the Lithium (ion) Li(+) correctly in the ionization energy.
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. In the Bohr model electrons have specific energy, and can occupy specific shells. Therefore the transition from one shell to an other is well defined, with discrete.
In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of.draw a Bohr-Rutherford diagram for lithium. draw a Bohr-Rutherford diagram for beryllium. draw a Bohr-Rutherford diagram for boron.
a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom. step 1. Bohr diagram of beryllium in addition bohr diagram of magnesium oxide furthermore n15 atom bohr diagram also diagram architecture plan stock photo along with pare argon radon furthermore electronic structure theories furthermore key diagram of power station also pare arsenic silicon together with drawing of lithium bohr diagram for 3 also.
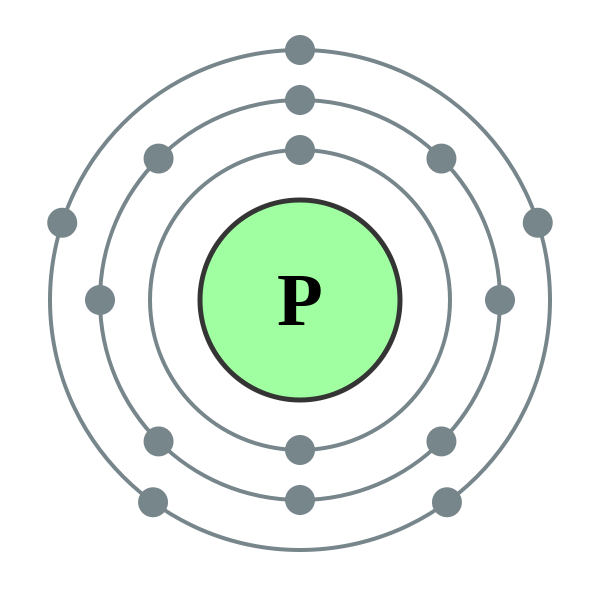
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second.
The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second.
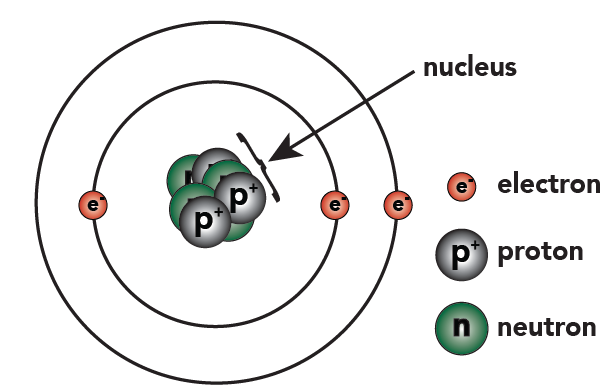
Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants. The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom.
Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.Lithium Bohr model | Science | ShowMeWhat is the Bohr model for Lithium