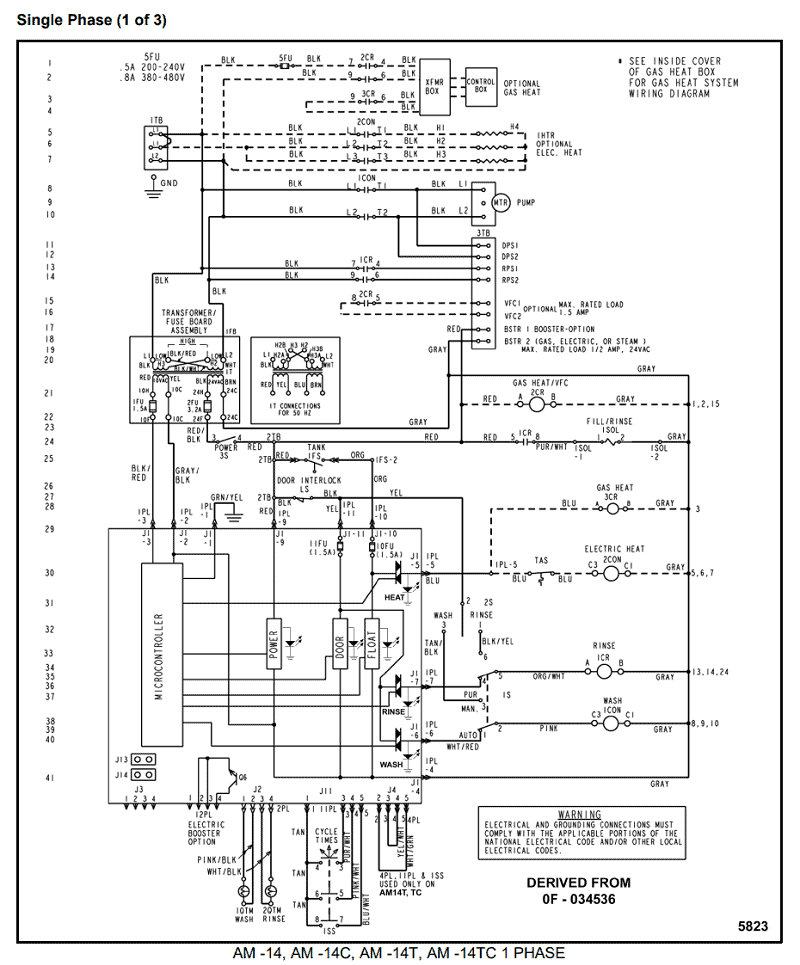
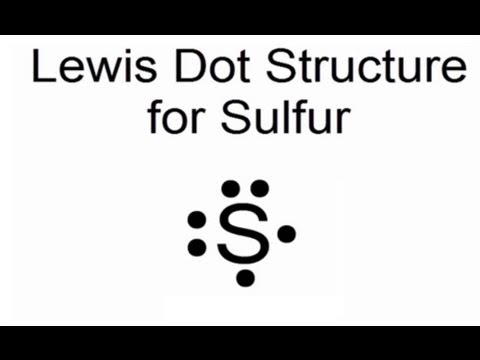
All About Sulfur!
Bohr Diagram. Picture.
Powered by Create your own unique website with customizable templates. Get Started.
A copper atom is a metal located in group period 4 of the Periodic Table of Elements. Its atomic symbol is Cu.
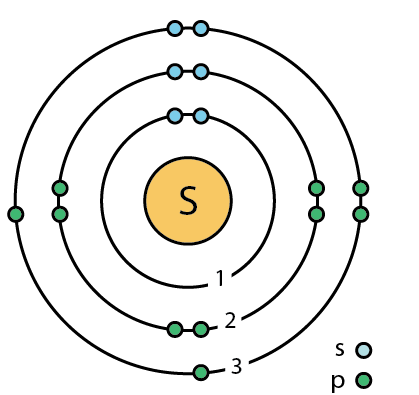
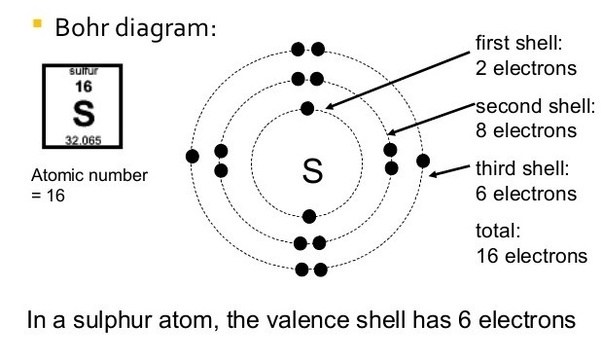
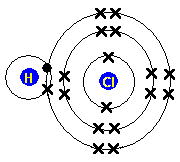
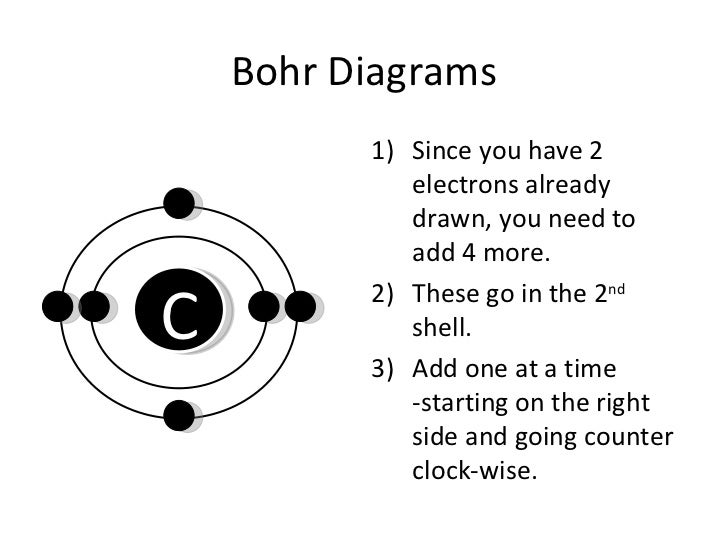
Each atom has 29 protons and electrons. The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three different energy levels, or orbits. Physics suggests that electrons do not.
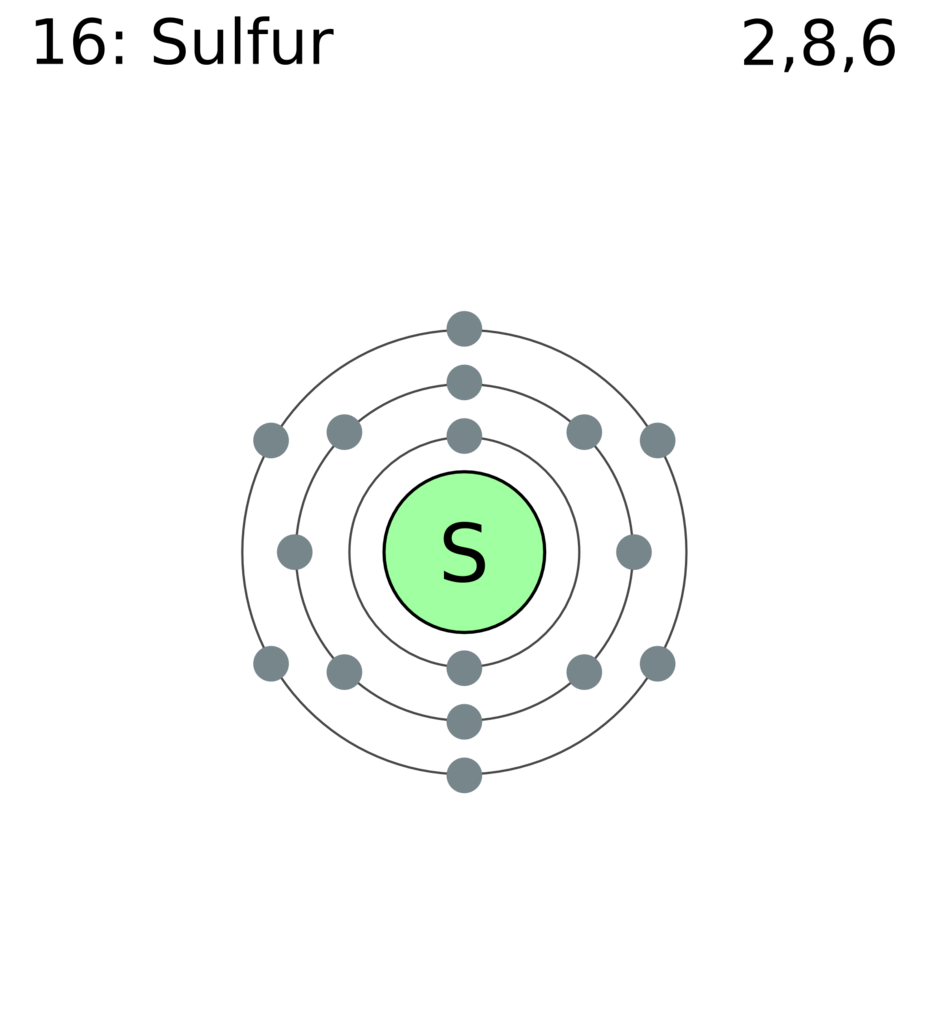
Here we go: 1st energy level – 2 electrons max 2nd energy level – 8 electrons max 3rd+ levels – 18 electrons max. Number of Protons/Electrons: Number of Neutrons: Classification: Non- metal.
Crystal Structure: Orthorhombic Density @ K: g/cm3. Color: yellow.What is the Bohr’s model for sulfur? How can you determine the Bohr diagram of gold?
Why is [1,3] sigmatropic shift of sulfur unlikely in the diagram below? What is the Bohr magneton?
What element can absorb sulfur? How many electrons does sulfur have?
Where was sulfur first used? Since bonding involves the. Element.
Bohr Diagram. Group.
Number. Lewis Dot Diagram Worksheet.
Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place.
Directions: Use the information provided for each element to draw Bohr Model diagrams. periodic table study guide how to draw bohr diagrams ppt bohr rutherford diagram best drawbacks rutherford model an sat chemistry acids bases and salts indicators 1 so2 sulfur dioxide molecular geometry & lewis structure geometry bohr diagram for calcium best ionic bonding explained what is an an overview on source rocks and the petroleum system of the central sulfur thinglink 17 elegant. Sulfur – 4.
Fluorine – 5. Calcium – 6.
Argon – Name ANSWER KEY Period Date Bohr Model Diagrams. Use the information provided for each element to draw Bohr Model diagrams. Label how many of each there are in the nucleus (e.g.
He: 2p, 2n). They should show all the electrons, 16 electrons in total.
There are three shells containing the electrons. The first shell has 2 electrons, next shell has 8 electrons and 6 electrons are in the outer shell.How to Make a Model of a Sulfur Atom | SciencingHow to Make a Model of a Sulfur Atom | Sciencing