In order to write the Calcium electron configuration we first need to know the we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom.

The atomic number of calcium is This means that in a neutral calcium atom, there are 20 protons in its nucleus. A neutral calcium atom also.
When we write the configuration we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Here I use the electron.
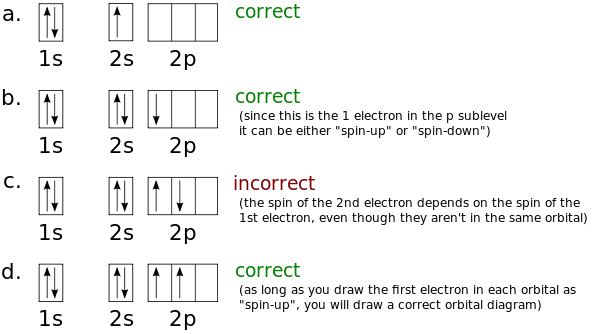
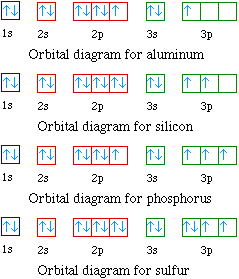
Orbital diagrams are pictorial representations of the arrangement of Rather than filling the orbitals one-by-one, it is easier to note that calcium is in the s- block. Yes, it is correct.
what type of picture do you need. On the internet there are a number of sites which give images of orbitals.
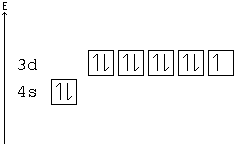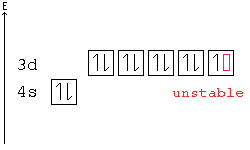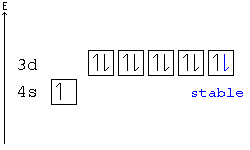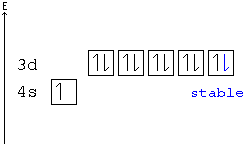
Just plug atomic.The s-orbital is always the lowest energy sublevel. Beyond the second energy level, the filling of atomic orbitals does not follow a simple pattern. For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels).
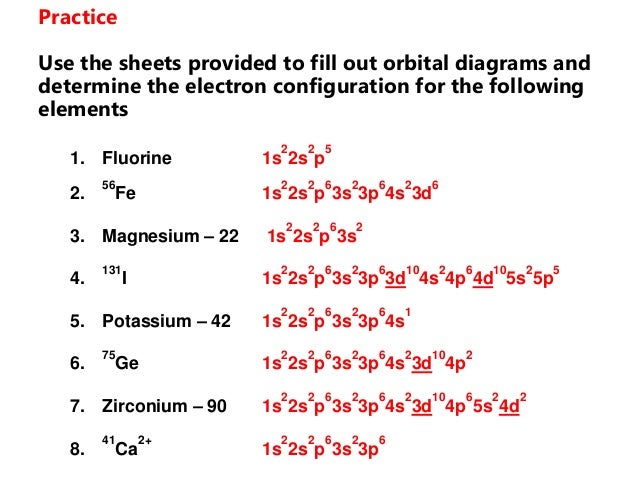
Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium.
The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital.
When we write the configuration we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Here I use the electron configuration chart to help us write the notation for Calcium.
Note that the last term in the Calcium electron configuration will be 1s2 2s2 2p6 3s2 3p6 4s2. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level.
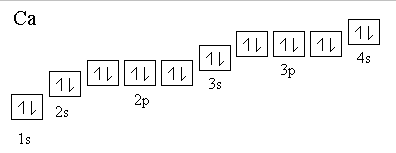
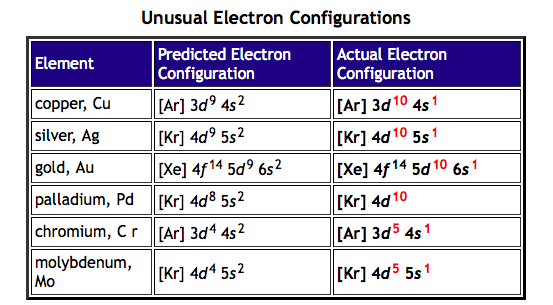
The oddity is the position of the 3d orbitals. They are shown at a slightly higher level than the 4s – and so it is the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.orbital filling diagram of calcium?
where can i find one? i need a picture? | Yahoo Answersorbital filling diagram of calcium?
where can i find one? i need a picture?
| Yahoo Answers