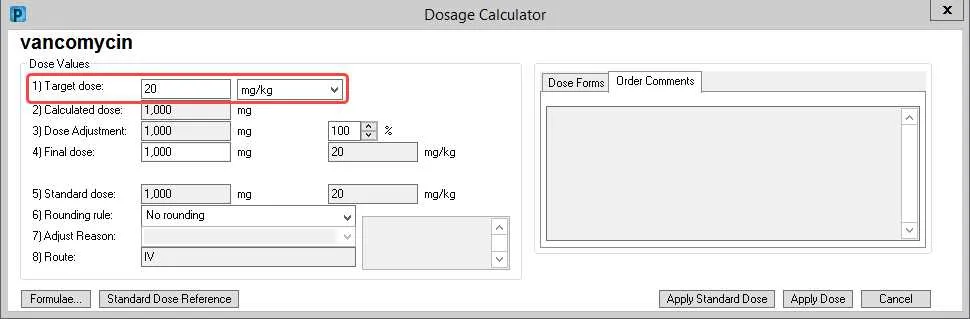
Precise modification of therapeutic amounts is essential for achieving maximal efficacy while minimizing adverse effects. Clinical protocols recommend tailoring intake based on individual patient factors such as renal function, hepatic metabolism, age, and concurrent therapies.
Stepwise titration frameworks enable systematic increments or reductions, improving safety margins especially in populations with narrow therapeutic indices. Utilizing a structured flowchart facilitates clear decision-making at each adjustment point.
Quantitative thresholds derived from laboratory markers or clinical parameters serve as benchmarks for altering treatment strength. Integrating these indicators into a clear visual tool supports healthcare providers in making evidence-based, timely revisions to treatment plans.
Medication Titration Chart
Modify the administration amount based on patient-specific parameters as follows:
- Renal Function:
- GFR > 90 mL/min: Standard amount
- GFR 60-89 mL/min: Reduce by 10-15%
- GFR 30-59 mL/min: Reduce by 25-30%
- GFR
- Hepatic Impairment:
- Mild dysfunction: Decrease by 10%
- Moderate dysfunction: Decrease by 25%
- Severe dysfunction: Avoid or reduce by 50%
- Body Weight:
- Under 50 kg: Reduce standard administration by 20%
- Above 100 kg: Increase by 15%, monitor closely
- Age Considerations:
- Over 65 years: Initiate at 75% of typical amount, titrate carefully
- Over 80 years: Start at 50-60%, monitor for adverse effects
- Drug Interactions:
- Concomitant CYP3A4 inhibitors: Lower quantity by 30%
- Concomitant CYP3A4 inducers: Increase by 20-25%
Always re-evaluate plasma levels or clinical markers within 3-5 days after modification to ensure therapeutic targets and minimize toxicity risk.
Interpreting Patient-Specific Factors for Dose Modifications
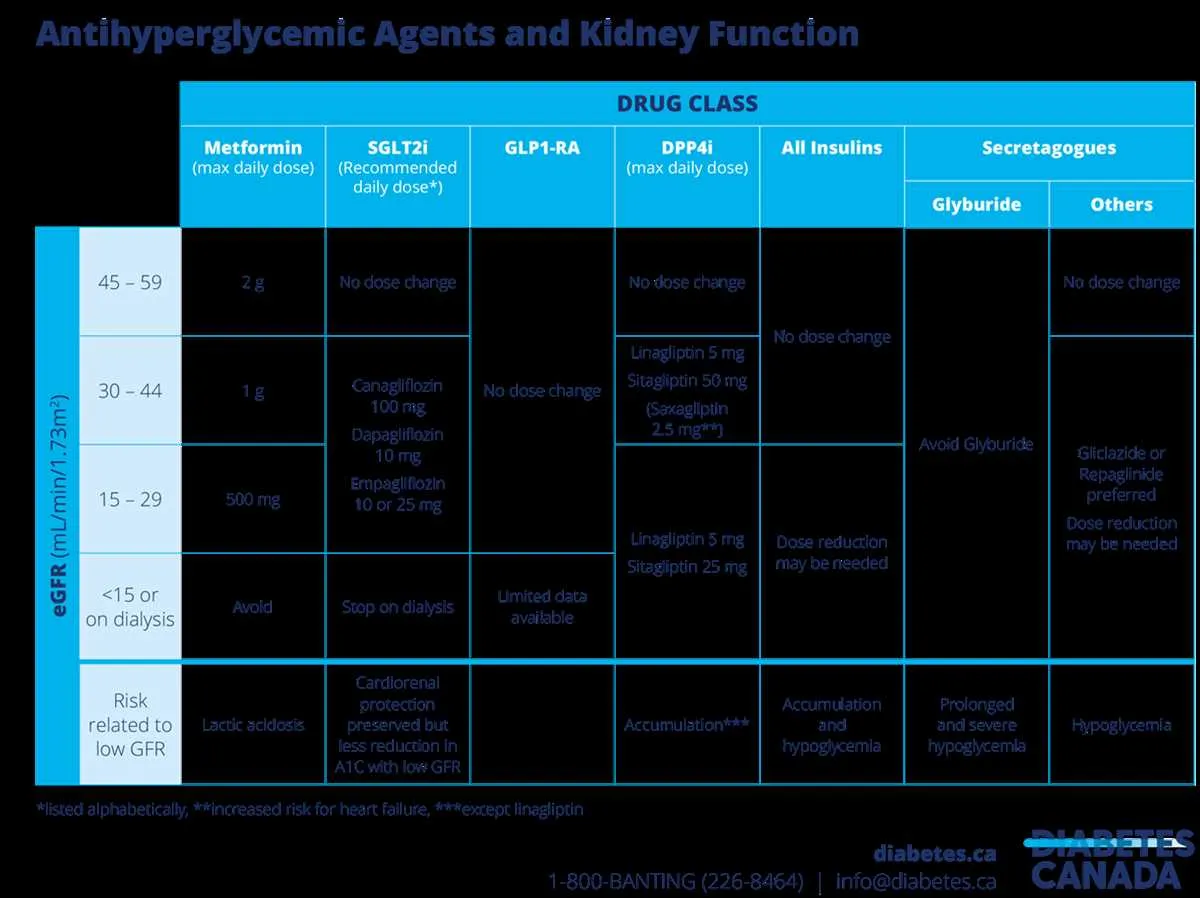
Base modifications primarily on renal and hepatic function assessments, as impaired clearance directly impacts therapeutic levels. For patients with estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73m², reduce administration by 50% or extend the interval by twice the standard period.
Age over 75 years often correlates with decreased metabolic capacity; initiate treatment at 25-50% of the standard amount and titrate cautiously. Monitor serum concentrations closely during the first week.
Body weight influences plasma concentration variability. For individuals under 50 kg, consider a 20-30% reduction to avoid toxicity, while patients exceeding 100 kg may require up to a 25% increase for efficacy.
Concomitant use of enzyme inhibitors such as ketoconazole or ritonavir necessitates cutting the initial quantity by at least 40%, due to decreased clearance and elevated systemic exposure.
Genetic polymorphisms affecting cytochrome P450 enzymes, especially CYP2D6 and CYP3A4, demand personalized scaling based on pharmacogenetic testing results. Poor metabolizers typically need halved amounts.
In cases of acute illness with fever or inflammation, expect altered pharmacokinetics; reduce the administered amount by 30% and reassess daily until stabilization.
For pediatric patients, utilize weight-based calculations (mg/kg) combined with age-specific metabolic rates rather than fixed quantities to ensure safe and effective plasma concentrations.
Utilizing Renal and Hepatic Function Metrics in Therapeutic Regimen Decisions
Base regimen modifications on creatinine clearance (CrCl) or estimated glomerular filtration rate (eGFR) for kidney function, and on Child-Pugh or MELD scores for liver impairment. For patients with CrCl below 30 mL/min, reduce systemic exposure by at least 50% or extend the interval between administrations. When eGFR ranges between 30-50 mL/min, consider a 25-50% reduction or more frequent monitoring of plasma levels.
In hepatic insufficiency, classify patients according to Child-Pugh classification: Class A (mild), Class B (moderate), Class C (severe). For Class B, decrease the amount or frequency of active agent by 25-50%. In Class C, avoid use if alternatives exist or reduce exposure by more than 50% with close monitoring of adverse effects.
Apply therapeutic drug monitoring (TDM) for compounds with narrow therapeutic windows, especially in cases of combined renal and hepatic dysfunction. Use plasma concentration targets to fine-tune administration schedules and prevent toxicity or subtherapeutic effects.
Adjust treatment regimens dynamically, repeating renal and hepatic function tests every 7-14 days in unstable patients or after acute events affecting organ performance. Employ formulas like Cockcroft-Gault for renal estimation and incorporate serum bilirubin, albumin, and INR for hepatic scoring to guide regimen modifications precisely.
Applying Dosage Modification Charts in Clinical Practice
Initiate patient treatment by referencing personalized titration schemes based on renal and hepatic function metrics. Modify administered quantities proportionally to creatinine clearance levels below 60 mL/min to prevent toxicity. For patients with liver impairment, decrease prescribed amounts by 25-50% depending on severity, as classified by the Child-Pugh score.
Integrate therapeutic monitoring data, such as plasma concentration measurements, to refine administration levels within the recommended therapeutic window. Adjust frequency and quantity according to observed serum levels, maintaining efficacy while minimizing adverse effects.
Use graphical protocols for rapid decision-making during acute phase reactions or concurrent use of interacting agents like CYP450 inhibitors, which may necessitate further reduction in treatment intensity. Apply caution when combining with medications that alter metabolic pathways or elimination routes.
Implement standardized clinical flowcharts in electronic health records for consistent evaluation and documentation of titration changes. Regularly reassess patient response and laboratory values to ensure ongoing appropriateness of the modified regimen, especially in elderly or polymorbid populations.