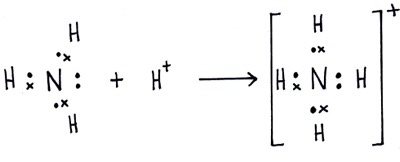
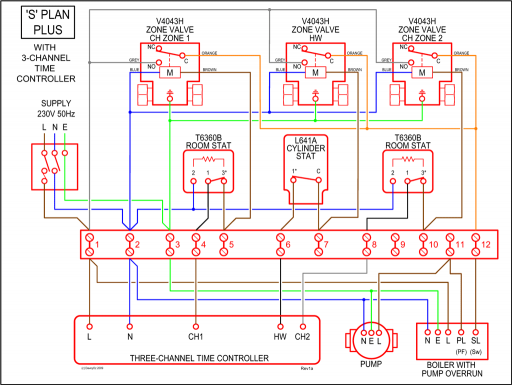
And 3×N−H bonds, and one nitrogen-centred lone pair of electrons. https://tse1.
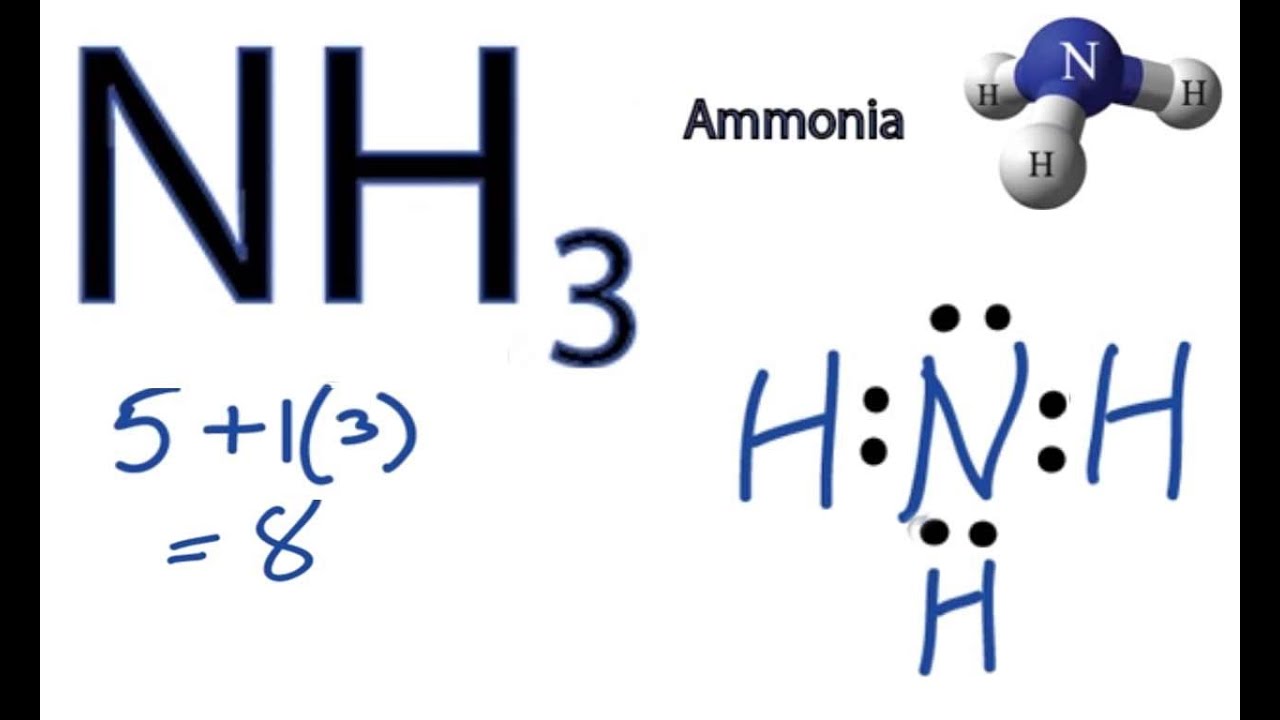
schematron.org?id= schematron.org?id=OIP. Ammonia has 4 regions of electron density around the central nitrogen atom (3 bonds and one lone pair).
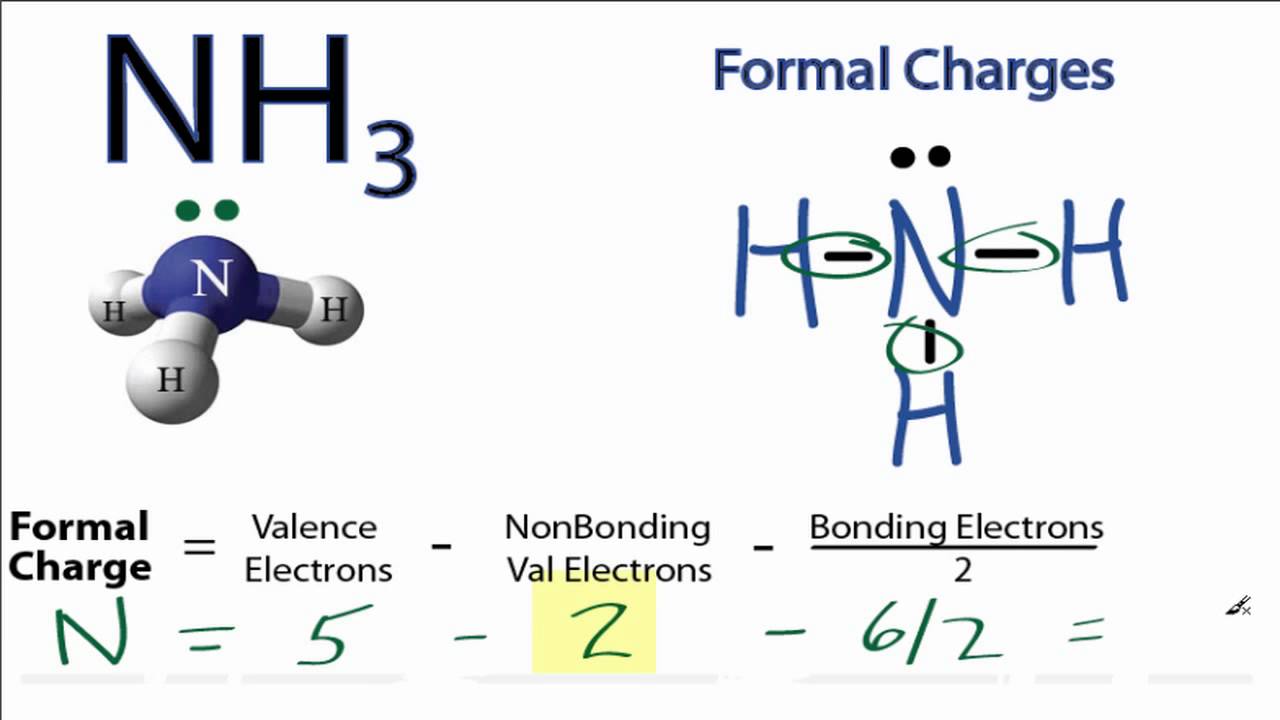
These are arranged in a tetrahedral shape. Electron Dot Structure of NH3 by Jeff Bradbury – February 17, – Lewis Electron Dot Structure for ammonia molecule NH3. You look up “electron dot diagram for ammonia” on google images.
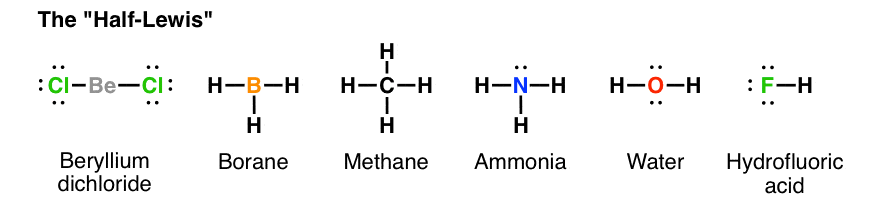
It really isn’t that hard. A step-by-step explanation of how to write the Lewis Dot Structure for NH3 ( Ammonia or Nitrogen Trihydride). The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom.
The N atom then has two dots on the unconnected side. NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
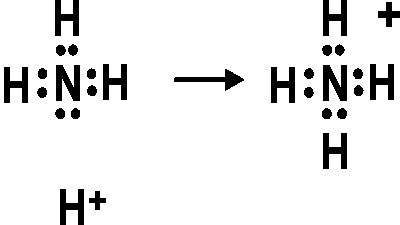
Each hydrogen atom is covalently bonded to the nitrogen via an electron pair, and another pair of electrons is attached to the nitrogen atom’s outer shell. The electron dot structure of NH3 has a nitrogen atom in the center.
It then is single bonded to three hydrogen atoms and has one lone pair. These arrange in a tetrahedral geometry. Use information from step 4 and 5 to draw the lewis structure.

Nitrogen goes in the centre. Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3.
Calculate the total valence electrons in NH3 molecule. N=5,H=1×3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. We’re going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride.
On the periodic table, Nitrogen is in group 5 or 15 so it has 5 valence electrons, and then Hydrogen is in group 1. It has one valence electron, but we have 3 Hydrogens, so let’s mutiply that by .VSEPR NH3 AmmoniaNH3 Lewis Structure [w/ free video guide]