Sigma (σ) bonding molecular orbital – Shared electron density is directly ..
Problem 6 – Use ethene (H2C=CH2) as a model to draw an MO diagram for ethanal. Bonding orbitals in Ethene (Ethylene) sp2.
sp2 hybrids. ethylene orbitals.
Jmol. which form the pi-bond.
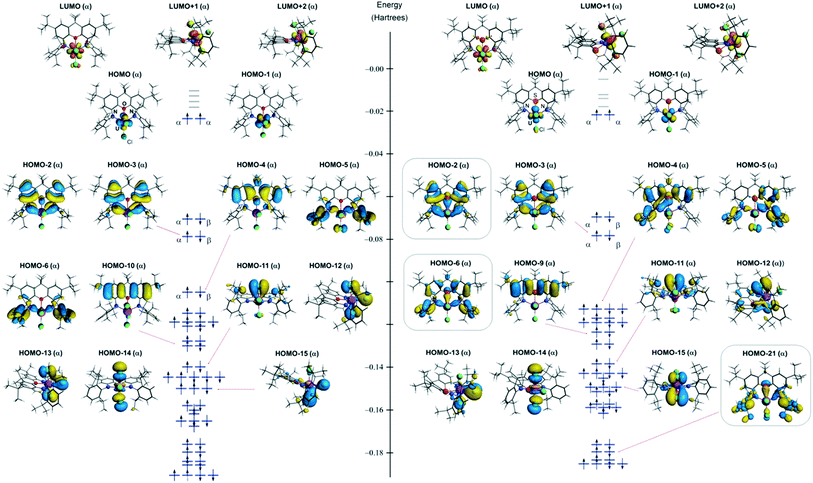
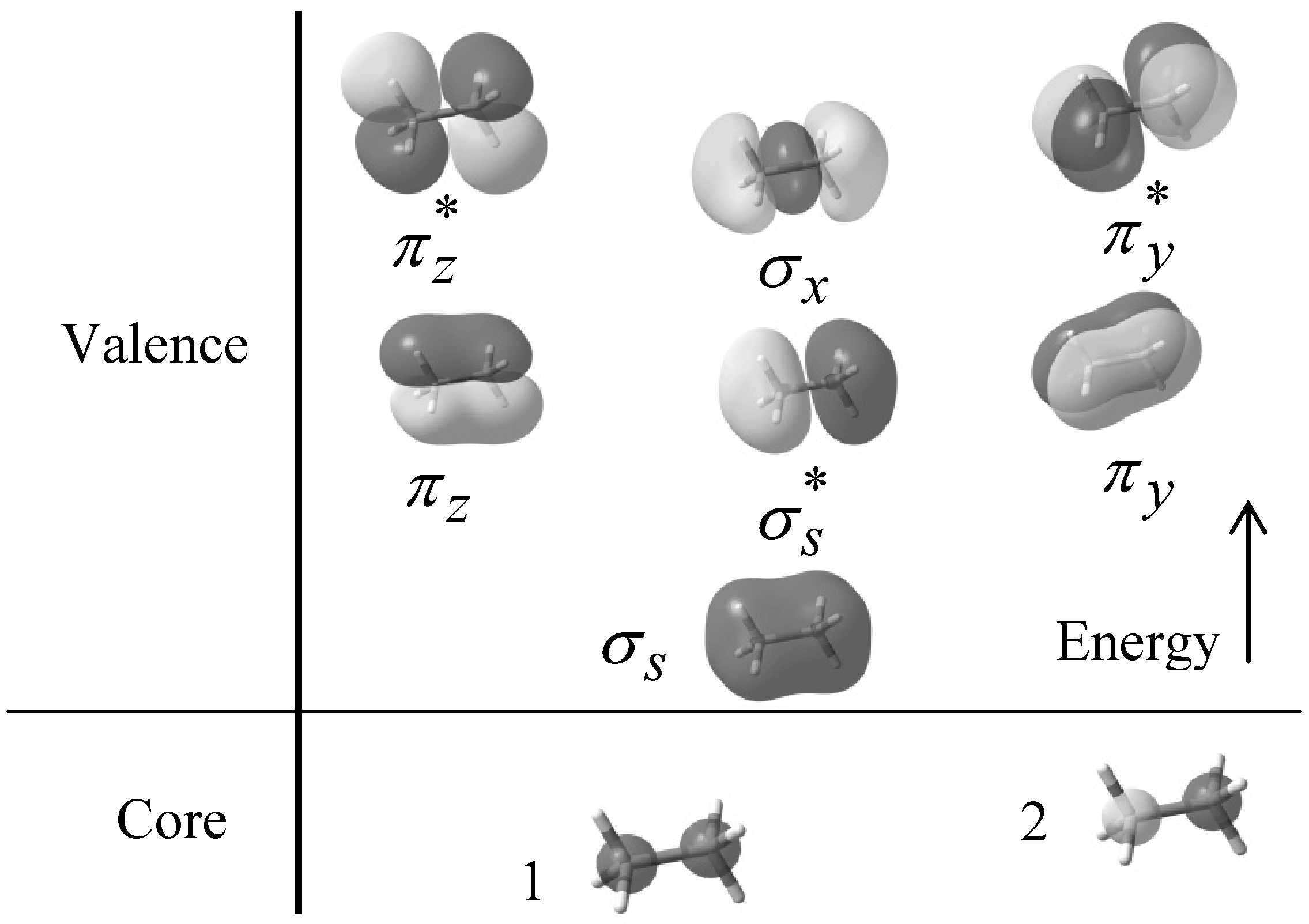
Explore bonding orbitals in other small molecules. Each line in this diagram represents one pair of shared electrons.
Ethene This sideways overlap also creates a molecular orbital, but of a different kind. In this.
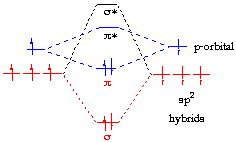
Bonding orbitals in Ethene (Ethylene) sp2. sp2 hybrids.
ethylene orbitals. Jmol.
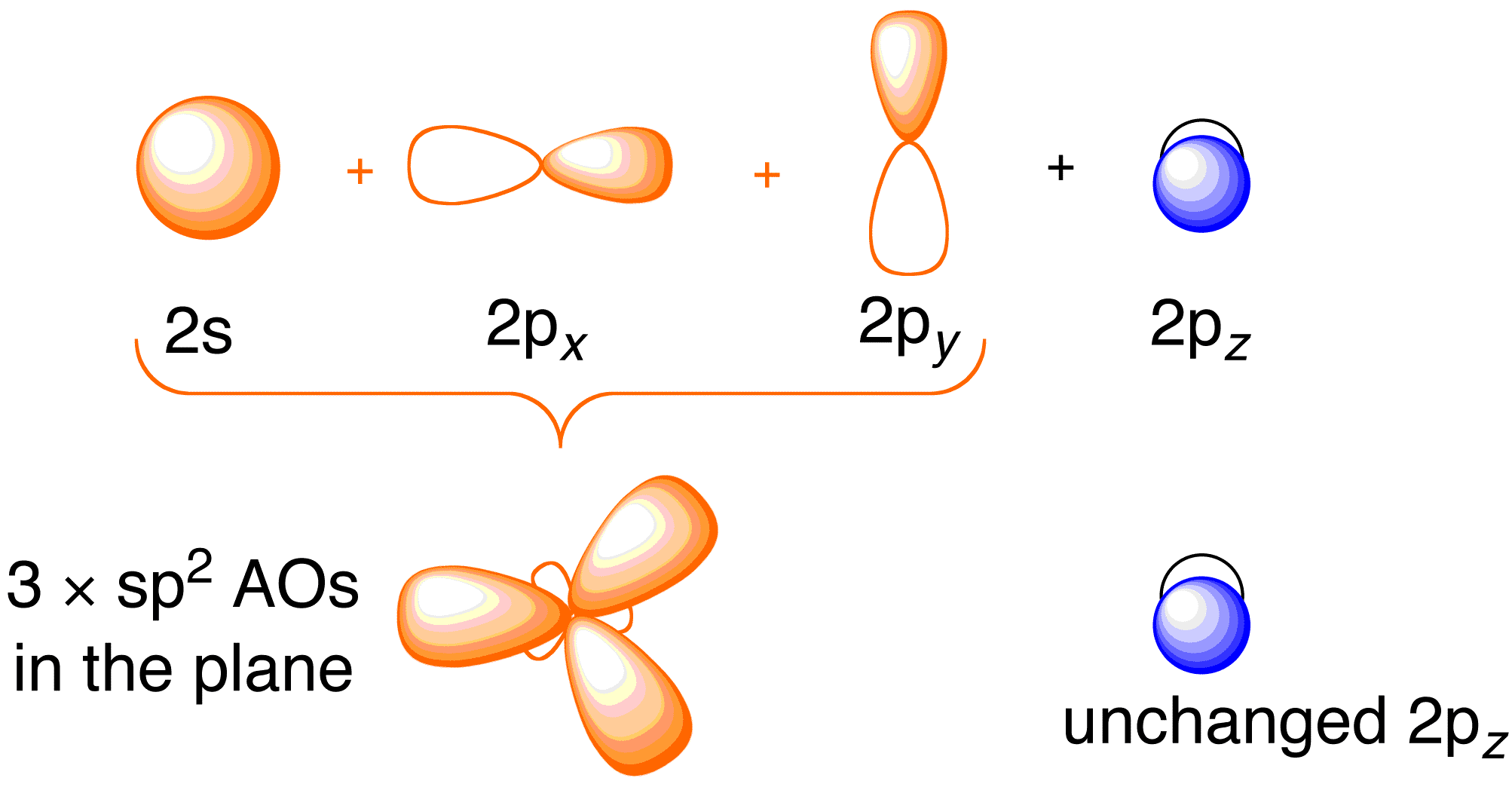
which form the pi-bond. Explore bonding orbitals in other small molecules. The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules.
This shows .Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond. At a simple level, you will have drawn ethene showing two bonds between the carbon atoms. Each line in this diagram represents one pair of shared electrons.

Ethene is actually much more interesting than this. An orbital view of the bonding in ethene.
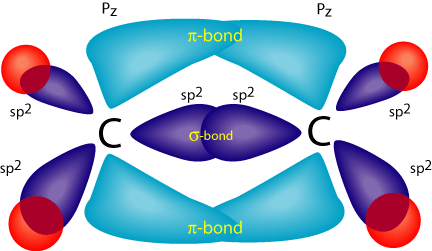
Ethene is built from hydrogen atoms (1s 1) and carbon atoms (1s 2 2s 2 2p x 1 2p y 1). A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below.
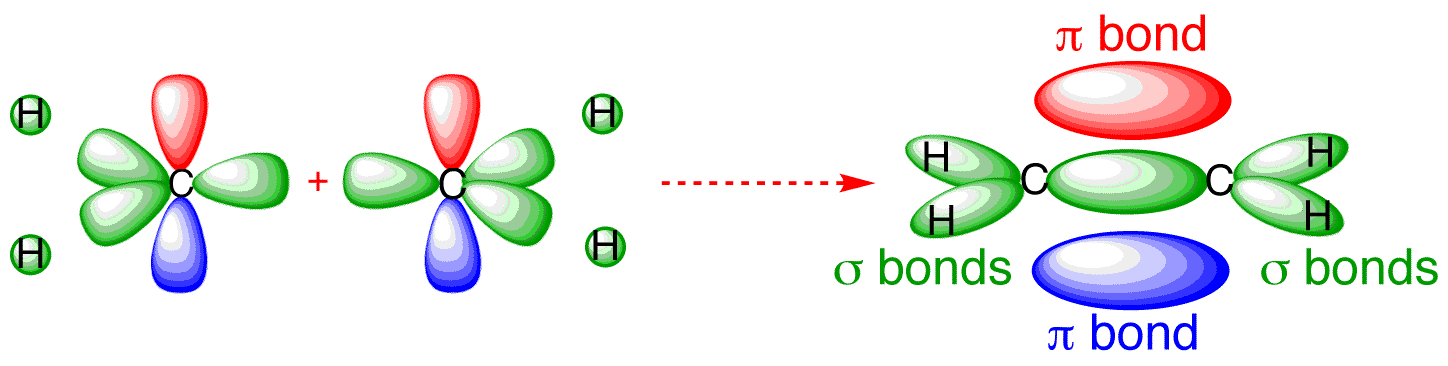
(McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. ) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two.
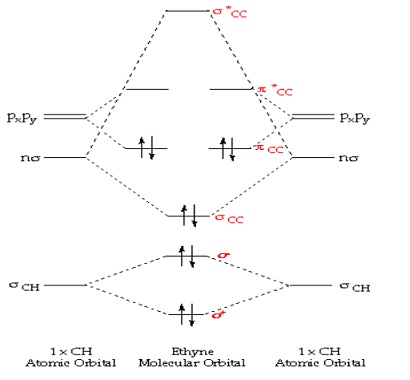
Ethene: The simplest alkene is ethene. Its chemistry is dominated by two “frontier orbitals”, that is the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO). For the ethene orbital energy diagram these are shown as p CC for the HOMO, and p * CC for the LUMO.
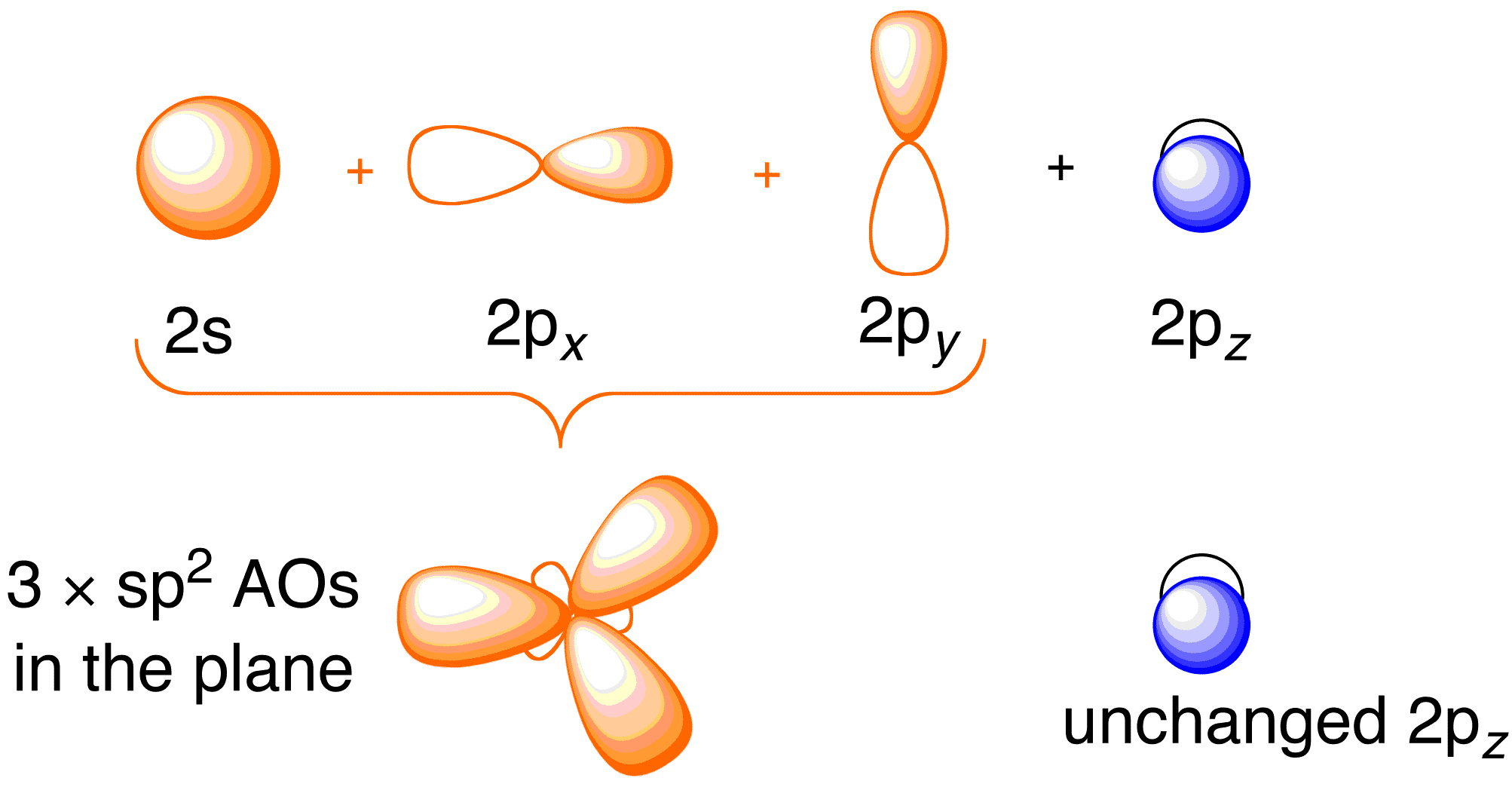
Do you notice something missing, broken, or out of whack? Maybe you just need a little extra help using the Brand.
Either way we would love to hear from you.π Molecular Orbitals of Conjugated ButadieneBonding orbitals in Ethylene (Ethene)