Exothermic Reaction. Endothermic Reaction.
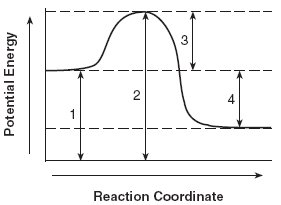
Reaction Coordinate Diagram. Energyproducts > Energyreactants.
Energyproducts < Energyreactants. A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction.
The ” reaction coordinate ” plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the.

One of your salts generated an endothermic reaction with water, while the other salt generated an . Energy diagrams for endothermic and exothermic reactions.Identify which points on sample reaction coordinate diagrams represent the activation energy and the change in energy Discuss what is plotted on the x- and y-axes of a reaction coordinate diagram.
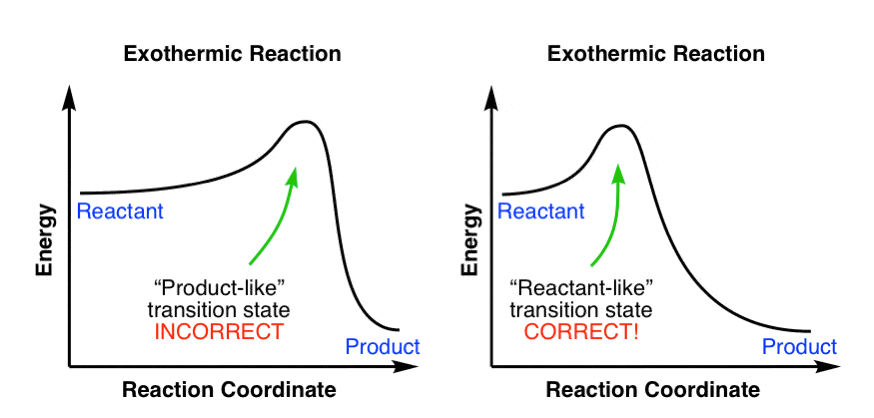
And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. A reaction coordinate diagram shows the energy changes that take place in each of the steps of the mechanism.
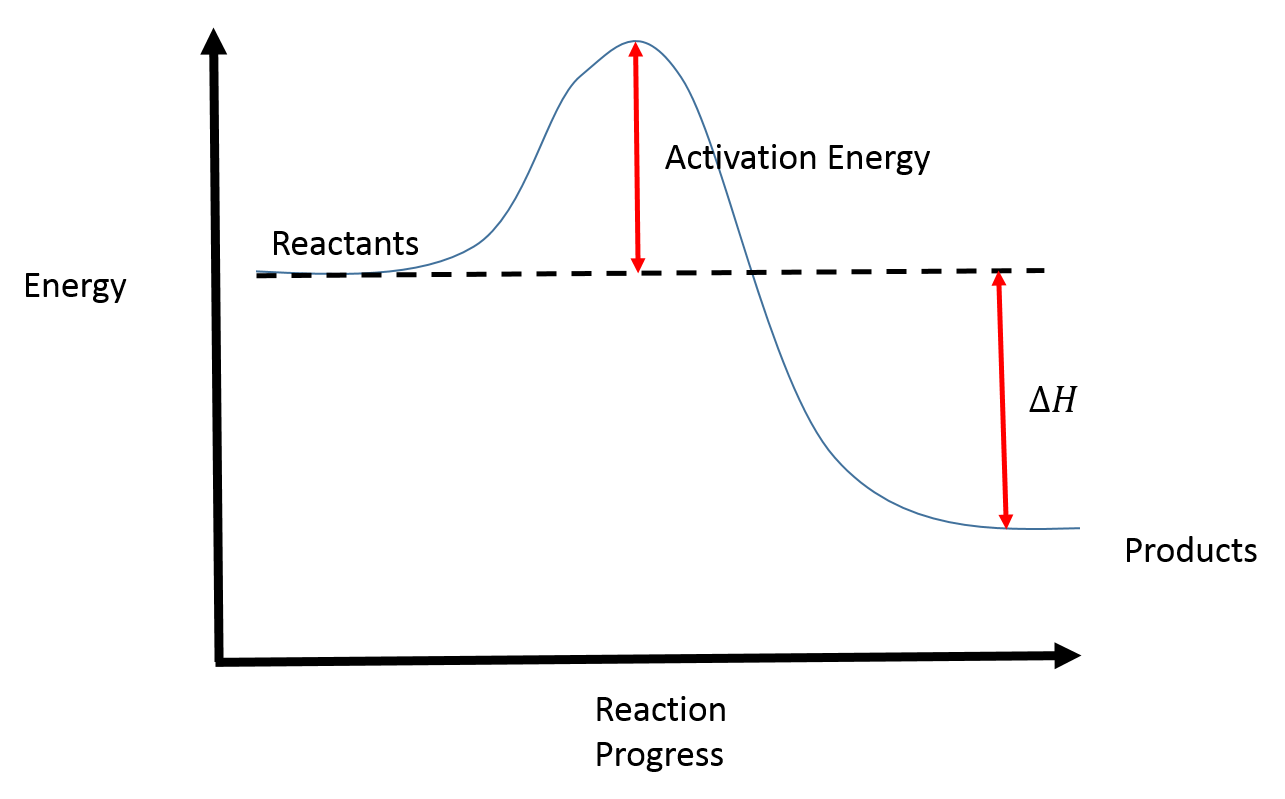
In a reaction coordinate diagram, the total energy of all species is plotted against the progress of the reaction. The reaction coordinate is a parametric curve that follows the pathway of a reaction and indicates the progress of a reaction.
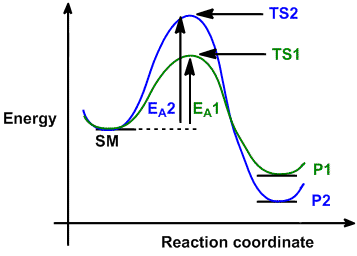
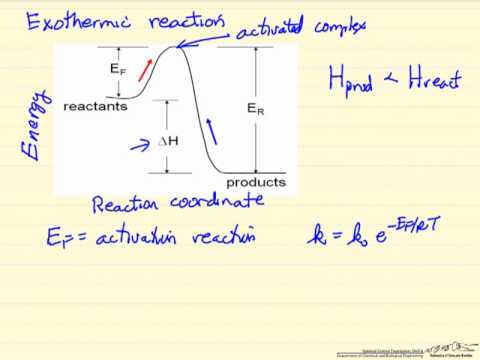
Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram.
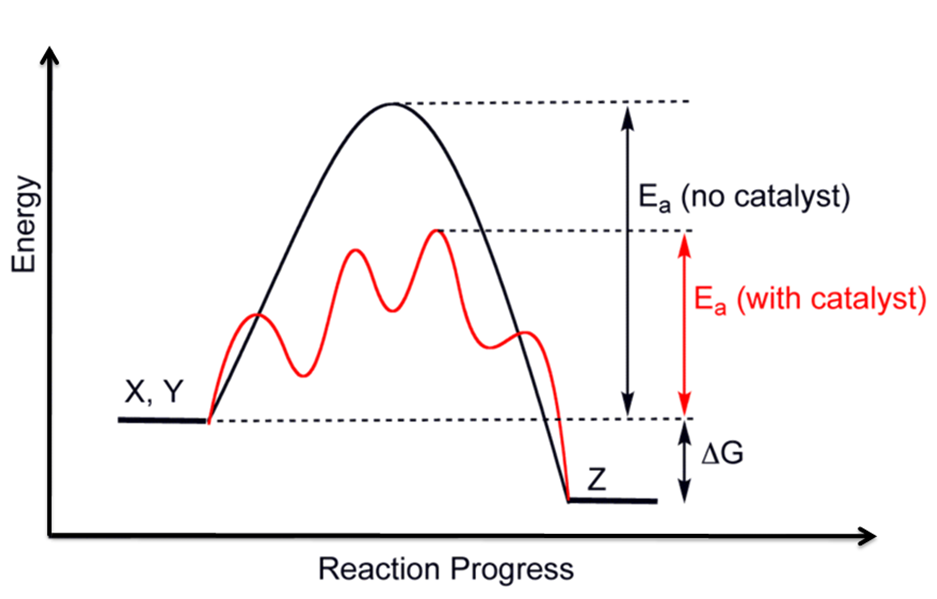
In other words, the products are more stable than the reactants. Overall Δ H ΔH Δ H for the reaction is negative, i.e., energy is released in the form of heat.Reaction Coordinate DiagramsEnergy profile (chemistry) – Wikipedia