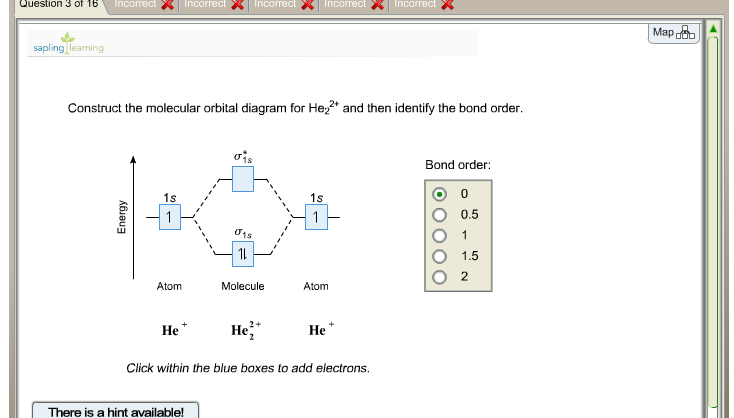
Solution: What is the bond order of He2+? a. 0 b.
½ c. 1 d.
1 ½ e. 2 Construct the molecular orbital diagram for H 2- a What charge would be needed on F 2 to.
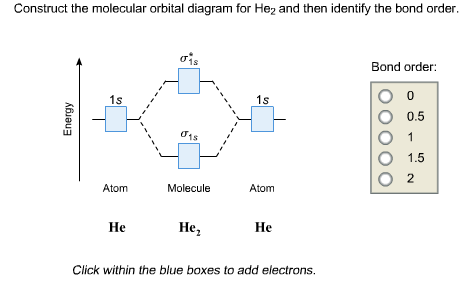
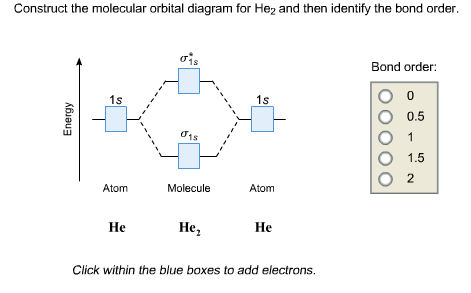
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.
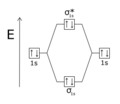
Figure \(\PageIndex{1}\): Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1s Atomic Orbitals. (a) The H 2 + ion.
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy.

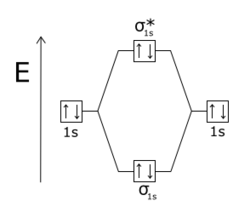
Draw this out using an energy level diagram: 2 He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. 2,.
H−. A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy.
Draw this out using an energy level diagram: 2 He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
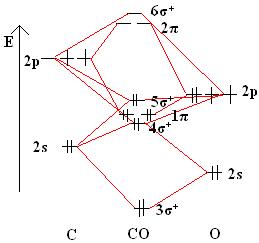
diagram for CO2 in Figure can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO 2 in Figure The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule.
Molecular orbital diagram
Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons – Number of Antibonding Electrons) = 1/2 x ( After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about times equilibrium down to bohr.
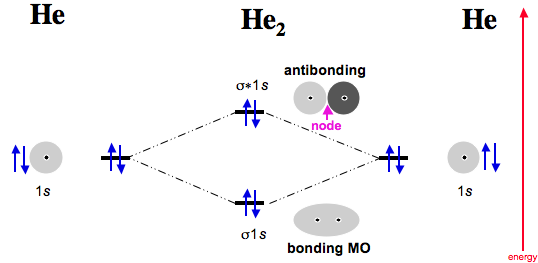
Molecular orbital correlation diagrams for He 2, He 2 +, N 2, N 2. The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.The Diatomic Helium Molecule – Chemistry LibreTextsMolecular Orbital Theory