
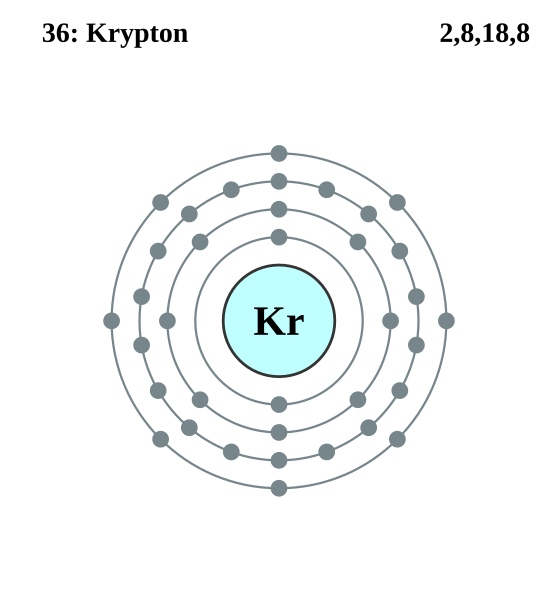
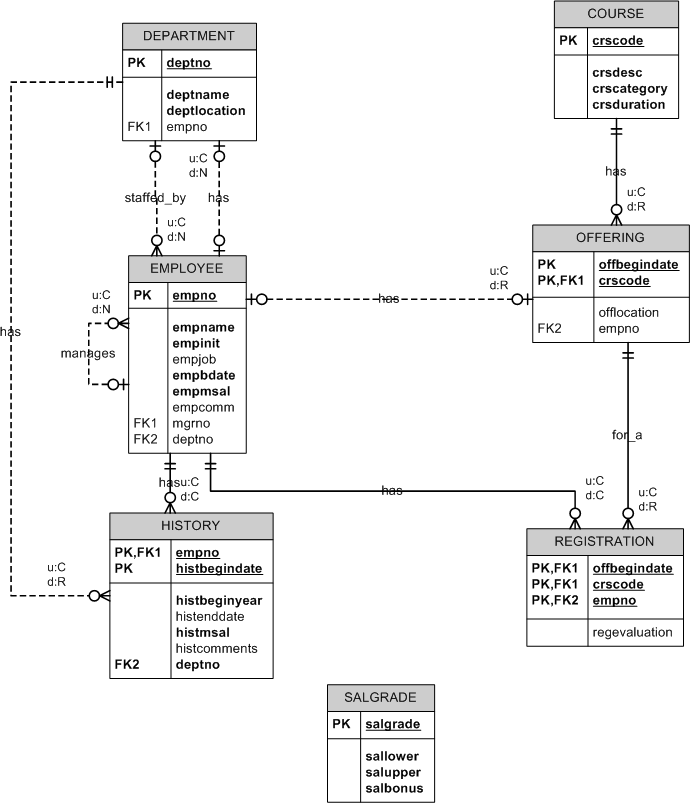
Krypton (the hidden element) is a noble gas, as such it valence shell is full and it is difficult to perform chemistry with it. electrons per.
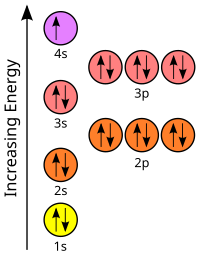
Draw orbital diagrams for the following elements: Full electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d2. Core notation: [Ar] 4s2 3d2.
7. krypton.
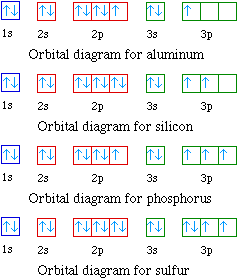
↑↓. ↑↓.
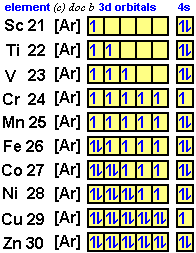
Since krypton is in the far right row of the periodic table, its outermost shell is full with eight electrons. This is one of the happy elements and has an electron configuration of The other inert gases including argon and xenon also have full outer shells with eight. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of krypton (atomic number: 36), the most common .
Start studying Unit 3: Orbital Diagrams. Electron configuration for Kr (Krypton).
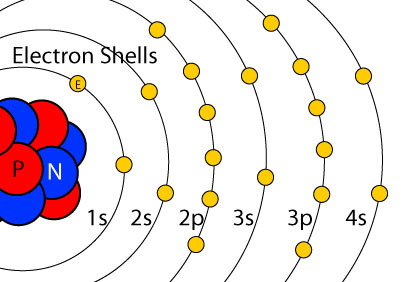
1s2 2s2 2p6 3s2 What element is represented by this orbital diagram?.In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital.

The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Krypton has a total of 36 electrons Second: KNOW YOUR ORBITALS Know how many electrons each orbital can hold and their order. (Refer to the following pictures as notes) Third: Write out the electron configuration For Krypton and most of the elements there are more the just one way (usually two) to write the electron configuration.

An orbital diagram naturally leads to the writing of an electron configuration. The electron configuration for chromium is: 1s22s22p63s23p64s23d4 The orbital diagram above is formatted in such a manner as to place the various orbital types at different energy levels. Orbital Diagrams.
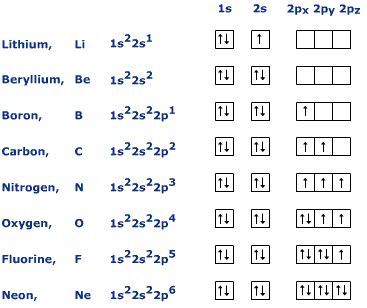
Many times it is necessary to see all the quantum numbers in an electron configuration, this the purpose of the orbital diagram. In addition to listing the principle quantum number, n, and the subshell, \(\ell\), the orbital diagram shows all the different orientations and the spin of every electron. Krypton (Kr) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.Krypton, atomic structure – Stock Image – C/ – Science Photo LibraryWhat is the Orbital diagram for krypton