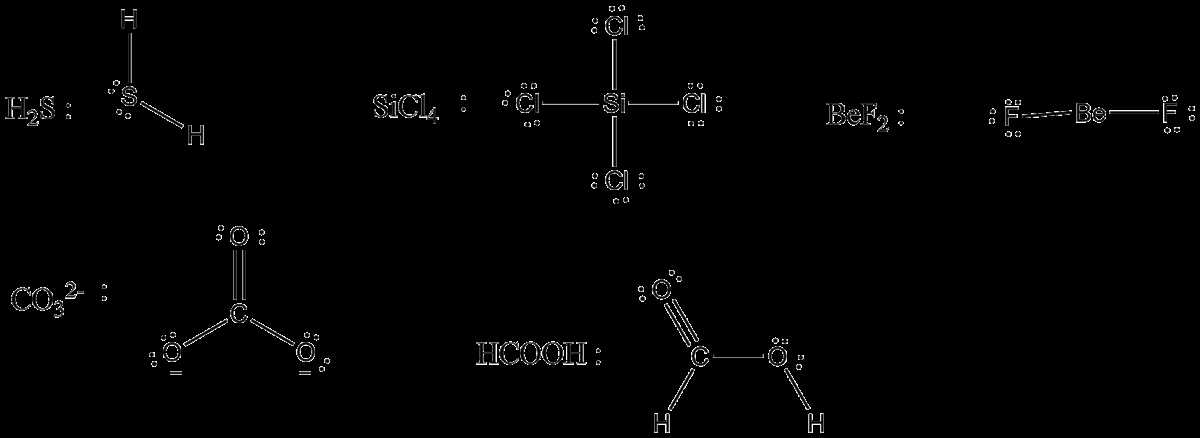
A Lewis diagram is a graphical representation of the valence electrons and the chemical bonds in a molecule or an ion. It was developed by American chemist Gilbert N. Lewis in the early 20th century. Lewis diagrams provide a simple and visual way to understand the electron distribution in a chemical species.
Chlorine, symbol Cl, is a halogen element found in group 17 of the periodic table. It has 17 electrons, including 7 valence electrons. To represent the Lewis diagram for Cl, we use the symbol Cl surrounded by dots, each representing a valence electron. The dots are placed around the symbol in pairs, with one pair on each side and one pair on top.
In the Lewis diagram for Cl, there are three pairs of dots placed on three different sides of the Cl symbol, with two dots in each pair. This represents the 7 valence electrons of Cl. The Lewis diagram shows that chlorine has a single unpaired electron, making it highly reactive and capable of forming chemical bonds with other atoms or ions.
Lewis Diagram for Cl
The Lewis diagram, also known as the Lewis structure or electron dot diagram, is a representation of the valence electrons in an atom or a molecule. It shows the arrangement of electrons around the atomic symbol, helping to understand the bonding and geometry of the molecule.
In the case of chlorine (Cl), it is a halogen element in group 7A of the periodic table. Cl has an atomic number of 17 and a valence electron configuration of 2s^2 2p^5. This means that chlorine has seven valence electrons, represented by dots around the Cl symbol in the Lewis diagram.
In the Lewis diagram for Cl, the Cl symbol is surrounded by seven dots, with one dot placed on each of the four sides (north, south, east, and west) and three dots placed on the top. These dots represent the seven valence electrons of chlorine. The Lewis diagram helps to visualize the electron arrangement and understand the potential bonding between chlorine atoms and other elements.
Chlorine is highly reactive and readily forms a covalent bond by sharing one electron with another atom to complete its valence shell. In a Lewis diagram for a chlorine molecule (Cl2), two chlorine symbols with their respective Lewis dot structures are placed next to each other, sharing a pair of electrons to form the covalent bond. The Lewis diagram for Cl2 would show a single line between the two chlorine symbols, representing the shared pair of electrons.
In summary, the Lewis diagram for Cl represents chlorine’s valence electrons as dots around the atomic symbol. It helps to understand the bonding and geometry of the molecule and shows how chlorine can form covalent bonds by sharing electrons with other atoms.
What is a Lewis Diagram?
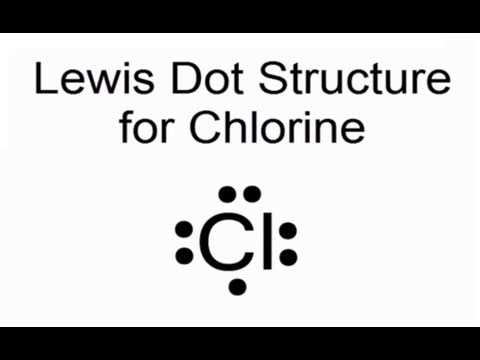
A Lewis diagram, also known as a Lewis structure or electron-dot diagram, is a representation of the valence electrons in an atom or a molecule. It was introduced by the American chemist Gilbert N. Lewis in 1916 as a way to depict the bonding and electron distribution in chemical compounds.
At its core, a Lewis diagram shows the arrangement of electrons in the outermost shell (valence shell) of an atom or molecule. Valence electrons are the electrons involved in chemical bonding and determining an element’s chemical properties. By using dots or lines to represent these electrons, a Lewis diagram provides a visual representation of the electron distribution and bonding patterns in a compound.
In the case of the Lewis diagram for Cl (chlorine), which has atomic number 17 and a valence electron configuration of 2s^2 2p^5, the diagram would show the seven valence electrons surrounding the chlorine atom. Each electron is represented by a dot, and the dots are placed around the chlorine atom’s symbol, with a maximum of two dots on each side before doubling up.
The Lewis diagram for Cl would look like this:
- Cl
- •
- •
- •
- •
- •
- •
This Lewis diagram accurately represents the electron configuration of a chlorine atom and provides valuable information about its chemical behavior and potential for forming bonds with other atoms.
Representation of Cl Atom
The representation of a chlorine (Cl) atom can be done using a Lewis diagram. A Lewis diagram is a visual representation that shows the valence electrons of an atom and how they are arranged in the atom’s outermost energy level. For chlorine, the electron configuration is 2-8-7, meaning it has 17 electrons in total with 7 valence electrons.
In a Lewis diagram, the symbol for the element is represented in the center and the valence electrons are drawn around it. Each valence electron is represented by a dot. For the chlorine atom, the symbol Cl is placed in the center and seven dots are drawn around it to represent the seven valence electrons. The dots are evenly distributed around the symbol, with no more than two dots placed on each side.
Table:
| Element | Symbol | Electron Configuration | Valence Electrons | Lewis Diagram |
| Chlorine | Cl | 2-8-7 | 7 | Cl • • • • • • • |
The Lewis diagram for a chlorine atom helps to illustrate its outer electron arrangement and can be used to understand its chemical properties. Chlorine is in Group 17 of the periodic table, also known as the halogens. It has a tendency to gain one electron to achieve a stable octet configuration, making it a strong oxidizing agent. The Lewis diagram provides a visual representation of this electron arrangement and its reactivity.
In conclusion, the representation of a chlorine atom can be visually depicted using a Lewis diagram, which shows the valence electrons arranged around the chlorine symbol. This diagram helps to understand the electron configuration and chemical properties of chlorine.
Electron Distribution in Cl Compound
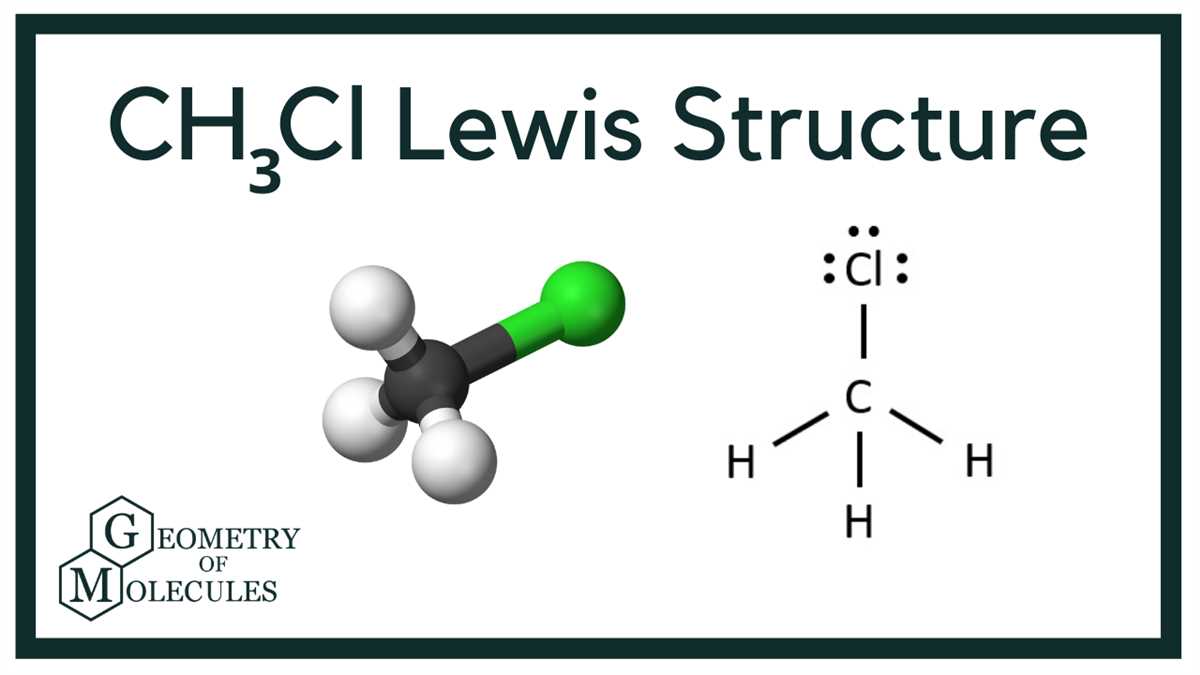
The electron distribution in a chlorine (Cl) compound is determined by the valence electrons of chlorine and the surrounding atoms or ions. Chlorine has seven valence electrons, and its electron configuration is 1s2 2s2 2p6 3s2 3p5. It is located in group 17 of the periodic table, also known as the halogens. Chlorine readily accepts one electron to achieve a stable octet configuration, resulting in an electron distribution of 2-8.
When chlorine combines with other elements to form compounds, such as chloride ions (Cl^-) or covalent compounds, it gains an electron to achieve a stable configuration. For example, in sodium chloride (NaCl), chlorine gains an electron from sodium to form the chloride ion. The Lewis diagram for chloride ion would show the chlorine atom with an additional electron represented as a single dot. The outermost electron shell would be full with eight electrons, fulfilling the octet rule.
Cl compounds can also form covalent bonds, where chlorine shares electrons with another atom. In this case, the Lewis diagram would show the chlorine atom with a single dot representing its unpaired valence electron, and the other atom connected through a line representing the shared electron pair. For example, in hydrogen chloride (HCl), the Lewis diagram would show chlorine with a single dot and hydrogen connected by a line, indicating the shared electron pair. The chlorine atom still achieves a stable configuration by sharing its unpaired electron.
In summary, the electron distribution in a Cl compound depends on whether it forms an anion by gaining an electron or forms a covalent bond by sharing electrons. In both cases, the chlorine atom aims to achieve a stable octet configuration by gaining or sharing electrons with other atoms or ions.
Applications of Lewis Diagrams
Lewis diagrams, also known as Lewis structures or electron dot structures, are widely used in chemistry to represent the valence electrons of atoms and the bonds formed between them. These diagrams provide a visual representation of the arrangement of electrons and help in understanding the structure, bonding, and chemical properties of molecules.
Here are some key applications of Lewis diagrams:
- Determining Molecular Geometry: Lewis diagrams can be used to determine the molecular geometry of a molecule. By counting the number of electron pairs around the central atom and applying the VSEPR theory, one can predict the shape and bond angles of the molecule.
- Predicting Bonding Patterns: Lewis diagrams allow us to predict the bonding patterns between atoms in a molecule. By examining the number of valence electrons and the octet rule, we can determine the type of bonds (single, double, or triple) that are likely to form.
- Understanding Reactivity: Lewis diagrams help us understand the reactivity of molecules by showing the availability of valence electrons and the potential for bond formation or breaking. This information is particularly useful in organic chemistry reactions and predicting the behavior of compounds in chemical reactions.
- Visualizing Resonance Structures: Lewis diagrams can illustrate resonance structures, which are multiple structural arrangements of a molecule that can be represented by different Lewis structures. Resonance structures are important in understanding the stability and delocalization of electrons within a molecule.
In summary, Lewis diagrams are essential tools in chemistry for understanding the structure, bonding, molecular geometry, and reactivity of molecules. They provide a simple yet powerful representation of electron distribution and facilitate the study and prediction of various chemical properties and behaviors.