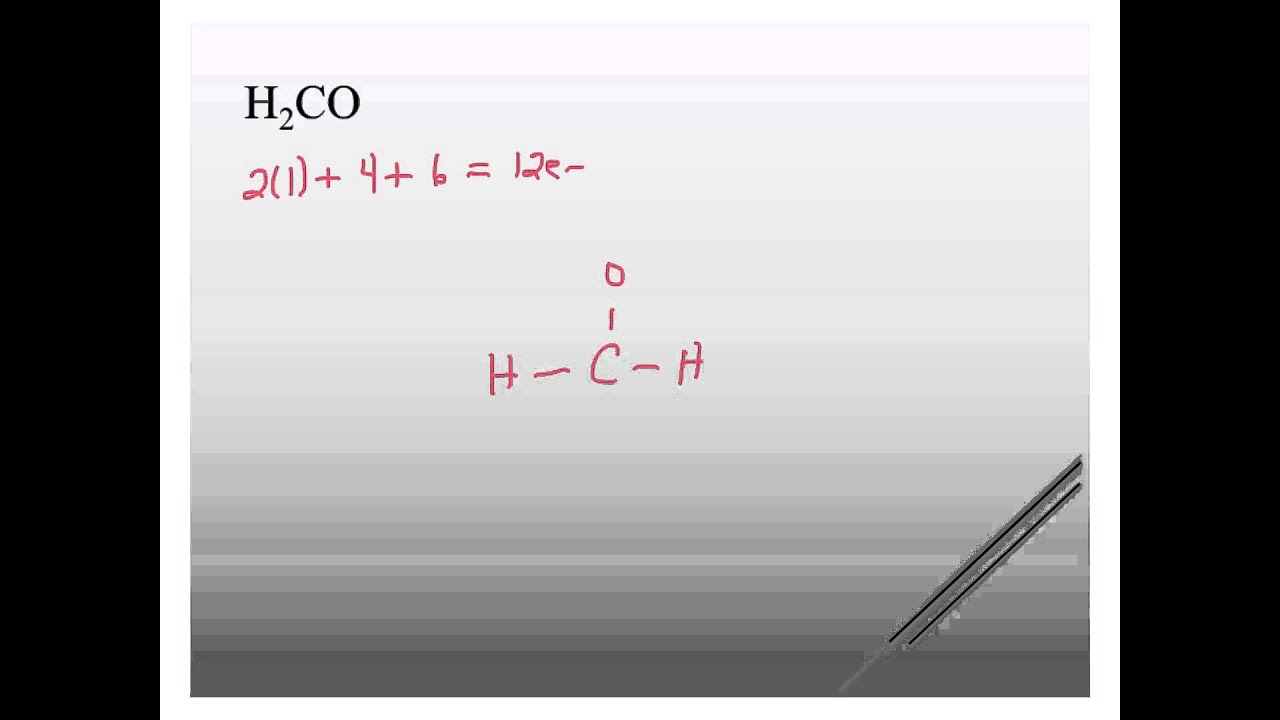
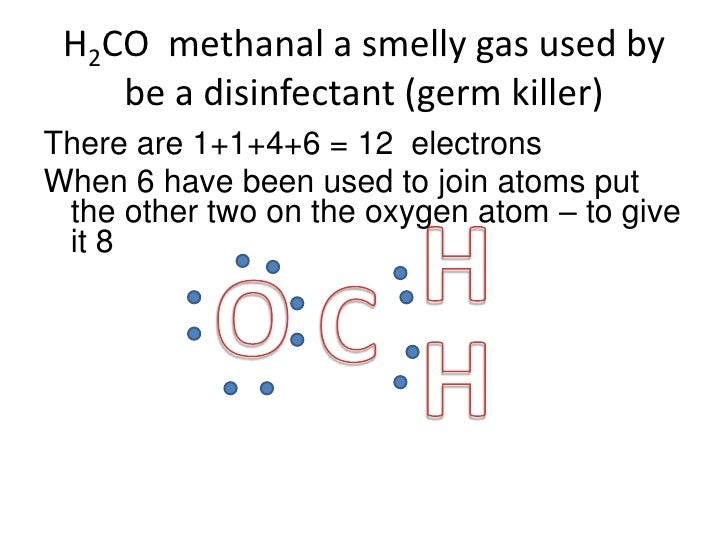
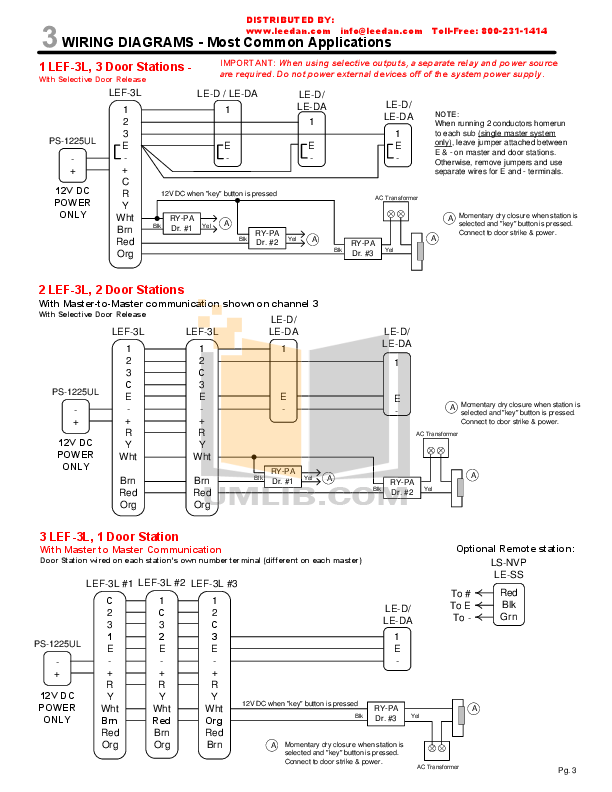
Solved: (a) Draw a Lewis diagram for formaldehyde (H2CO), and decide the hybridization of the central carbon atom. (b) Formulate the molecular orbitals for the.
first, count the TOTAL number of valence electrons second, identify the central atom, and write it with the other atoms around it. put the electrons.
(a) Draw the Lewis structure (electron dot diagram) for each of the following H2CO. Lewis structure. Approximate bond angle around the central atom. o.
Drawing the Lewis Structure for H2CO. Viewing Notes: There are a total of 12 valence electrons in the H2CO Lewis structure.
H2CO is also called Formaldehyde. Formaldehyde | H2CO or CH2O | CID – structure, chemical names, from CAMEO Chemicals, DOT Emergency Response Guidebook, NJDOH RTK.Use information from step 4 and 5 to draw the lewis structure.
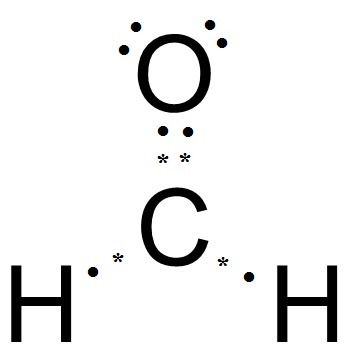
Carbon goes in the schematron.org sure carbon and oxygen get 8 electrons to fulfil octet rule. Lewis dot structure of H 2 CO.
Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule.
H:1×2=2 C:4 O Total= b Draw the Lewis structure for H2CO. By knowing the Lewis structure, we can also predict the three-dimensional geometry of an individual molecule.
Lewis symbols Covalent bonds Electronegativity Lewis Structures Part 1 Lewis . What Is the Lewis Dot Structure for H2CO?
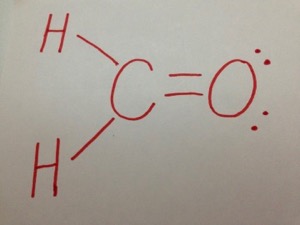
According to schematron.org, the Lewis dot structure for H2CO, or formaldehyde, is drawn by placing a C (carbon) in the middle, putting one H (hydrogen) on either side and putting an O (oxygen) on top. This configuration contains 12 valence electrons arranged.
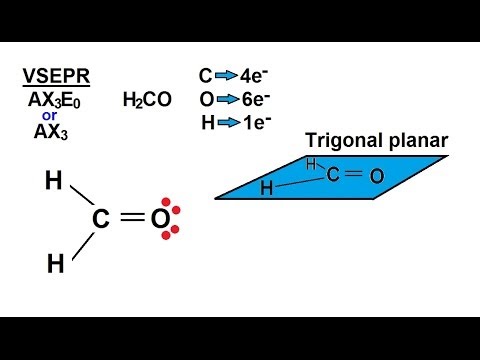
Formaldehyde is readily soluble in water and is commonly distributed as a 37% solution in water; formalin, a 10% solution of formaldehyde in water, is used as a disinfectant and to preserve biological specimens. Environmentally, formaldehyde may be found in the atmosphere, smoke from fires, automobile exhaust and cigarette smoke. What Is the Molecular Geometry of H2CO?
Formaldehyde, also known as H2CO, has trigonal planar geometry. This molecule consists of two single-bonded hydrogens attached to a carbon center that also has an oxygen double bond attached to it. The three atoms .Formaldehyde | H2CO – PubChemH2CO Lewis Structure – How to draw the Electron Dot Structure for H2CO