
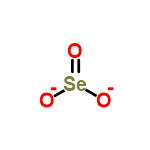
(1 points) How many electrons would be included in the Lewis dot structure of ( 2 points) Draw the Lewis dot structure for the polyatomic ANION selenite, SeO3. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . The structure and arrows are given, simply add the remaining bonds and lone.

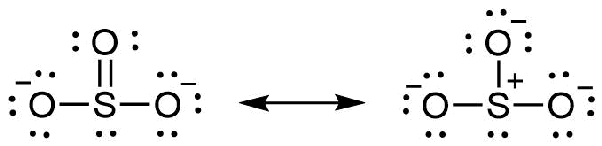
Answer to SeO3 lewis diagram, e- tally, shape and bond angle. According to the 1st Lewis structure it does because each O atom has a double bond with the central Se atom.

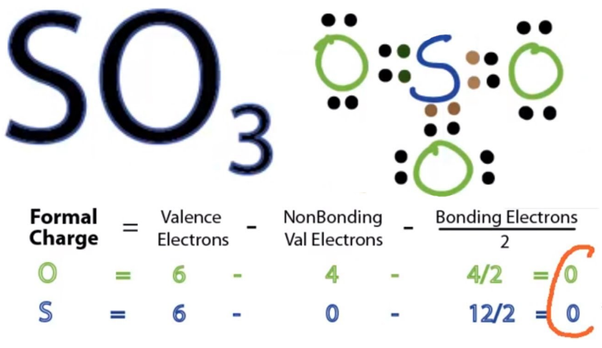
This gives you 12 valence. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O.
The Os.Clean Air Systems buys a diagnostic piece of equipment for $, The machine will be depreciated on a straight-line basis for 10 years with a salvage value 6. MT makes small camping and snowmobile trailers. The demand for camping trailers occurs between January and June (mostly in April and May.
The Lewis structure for SeO 2 has 18 valence electrons available to work with. SeO 2 is a Lewis structure with Selenium (Se) which can hold more than 8 valence electrons. It’s a good idea to check the formal charges for your SeO 2 Lewis structure to make sure they are zero.
SeO3 2. AsH 3 3.

NO2 – 4. BeFCl LEWIS DIAGRAM SHAPE BOND ANGLE POLAR OR NONPOLAR MOLECULE 5.

SiH2 Br2 6. SeH2 7.
PF5 8. SCl6 LEWIS DIAGRAM SHAPE BOND ANGLE POLAR OR NONPOLAR Predicting Molecular Geometry Part II: In each case, predict the approximate bond angle(s), around the central atom (Note: It is helpful to first sketch the Lewis stucture!). A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O.
Welcome to our website, we try to bring you relevant images to what you are looking for about “S2 Lewis Diagram”. Therefore we present the picture gallery below.

.Selenium trioxide | SeO3 – PubChemSO3 Molecular Geometry, Lewis Structure, and Polarity Explained