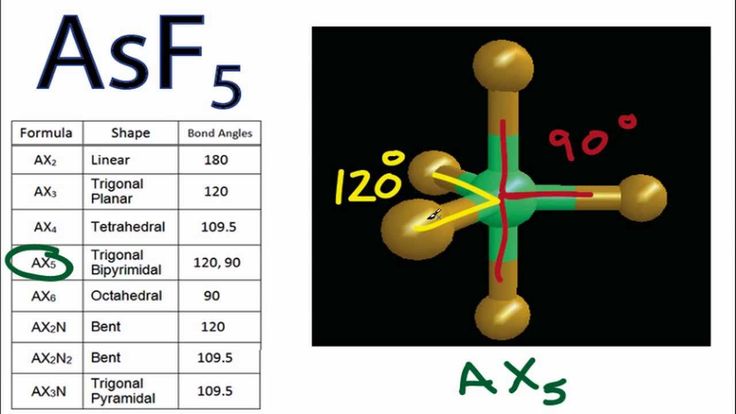
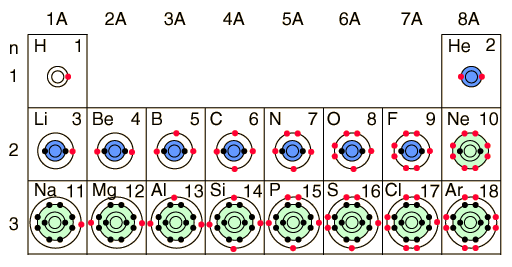
DRAWING LEWIS DOT DIAGRAMS 1) Given a Bohr model, count the 2 Mg DRAWING ELECTRON DOT DIAGRAMS Draw the Dot Diagram for arsenic (As). Comprehensive information for the element Arsenic – As is provided by this page including scores of Atomic Structure of Arsenic Electron Dot Model.
The left diagram shows a Lewis dot structure of sodium with. Likewise, they .. A Lewis structure shows an arsenic atom single bonded to three fluorine atoms.
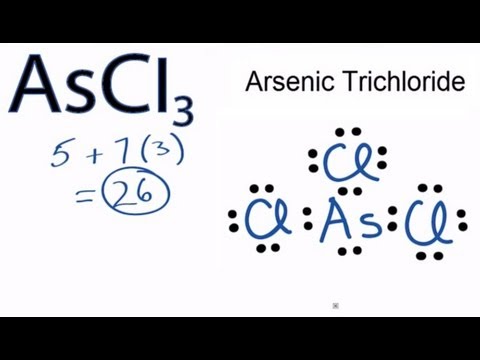
Arsenic is isoelectronic with nitrogen (they are both Group V elements), so there are 5 valence electrons. Arsenic cation (3+) | As+3 | CID – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.A compound with a molar mass of about 28 g/mol contains % carbon and % hydrogen by mass. Write the Lewis structure for a molecule of the compound.
A compound with a molar mass of about 42 g/mol contains % carbon and % hydrogen by mass. Write the Lewis structure for a . There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram.
To make the electron dot diagram you put the electron symbol and put a dot on o ne of the sides for each period (you don’t count the middle section.)Ne: = Neon dot diagram ‘ ‘. U.S. Department of Transportation (DOT) Identification number and the corresponding Guide number.

The DOT ID is also the UN Number”>. More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook.
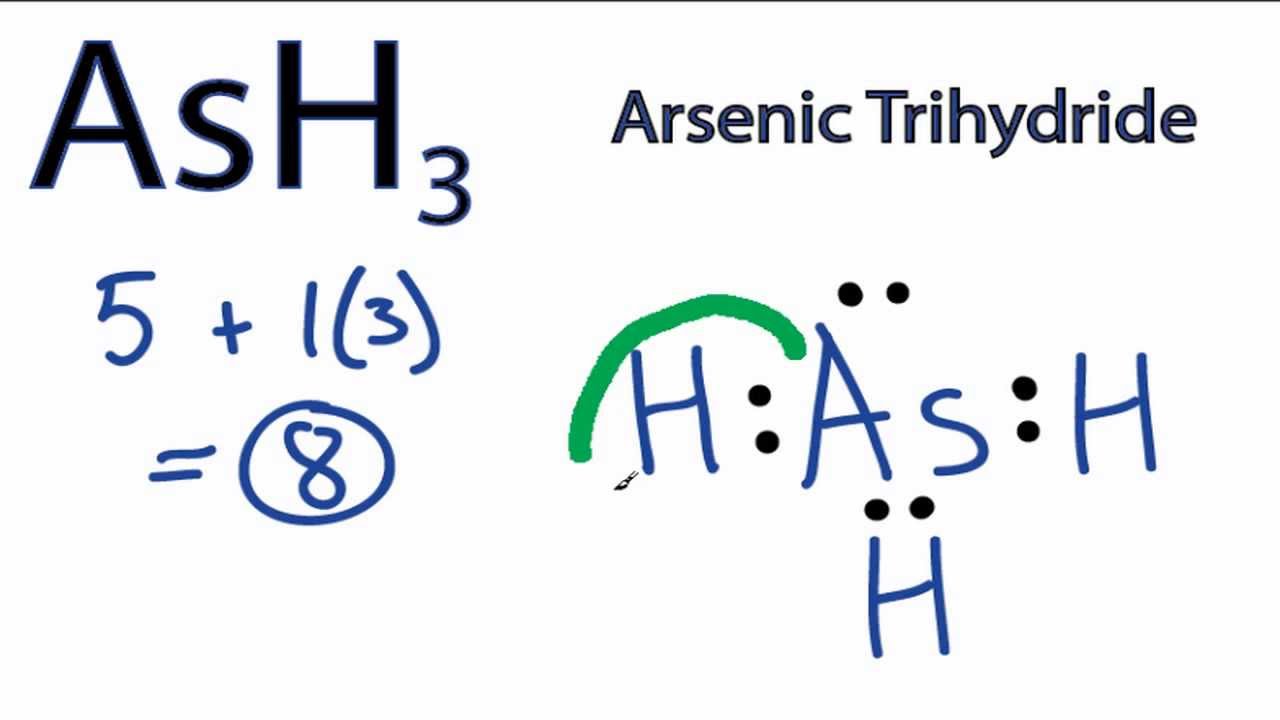
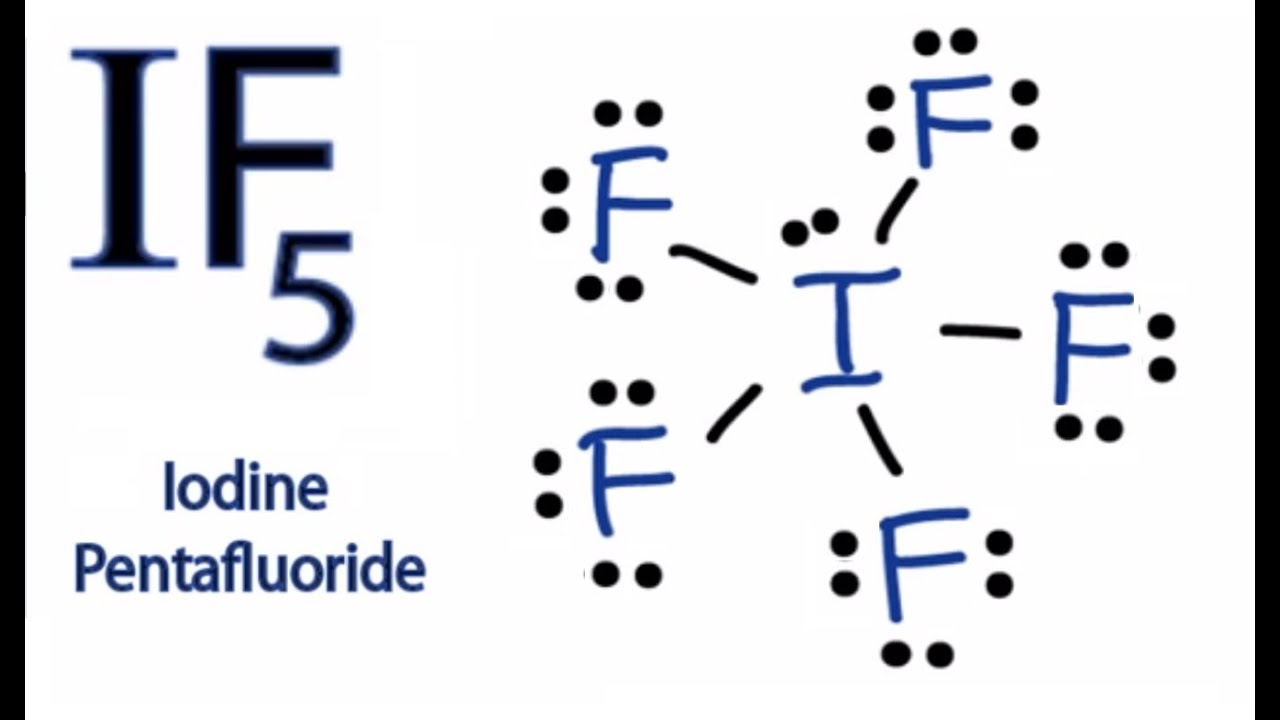
Transcript: This is the AsH3 Lewis structure: Arsenic Trihydride. As goes at the center, Hydrogens always on the outside.
We have three of them. For the AsH3 Lewis structure, we have 5 valence electrons for As, plus 1 for Hydrogen, but we have 3 Hydrogens, for a total of+ valence electrons. Every chemistry student has to learn how to draw Lewis Dot Structures.
The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule.
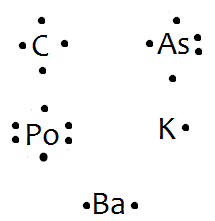
how the molecule might react with other molecules. the physical properties of the molecule (like boiling point, surface tension, etc.).Electron (Lewis) Dot Diagrams – SiverlingWhat is the dot diagram for arsenic