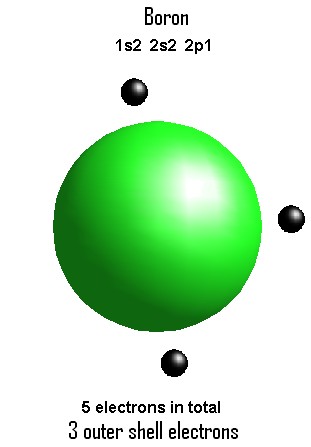
The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Because experiments suggest that there is some degree of overlap.
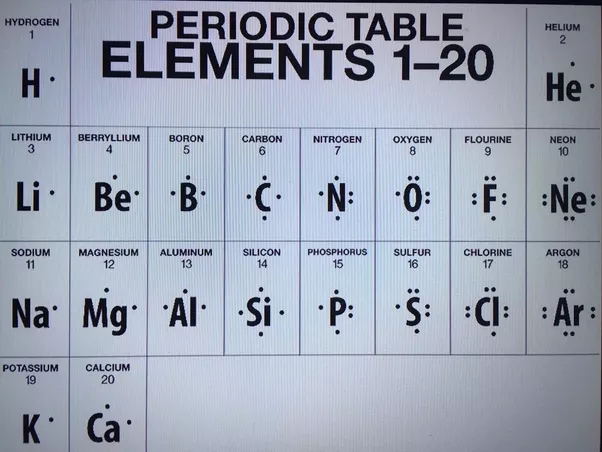
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical dot diagram is like that of helium: The next atom is boron.
Structure, properties, spectra, suppliers and links for: Boron nitride. Describe the electron dot diagram system of representing structure.
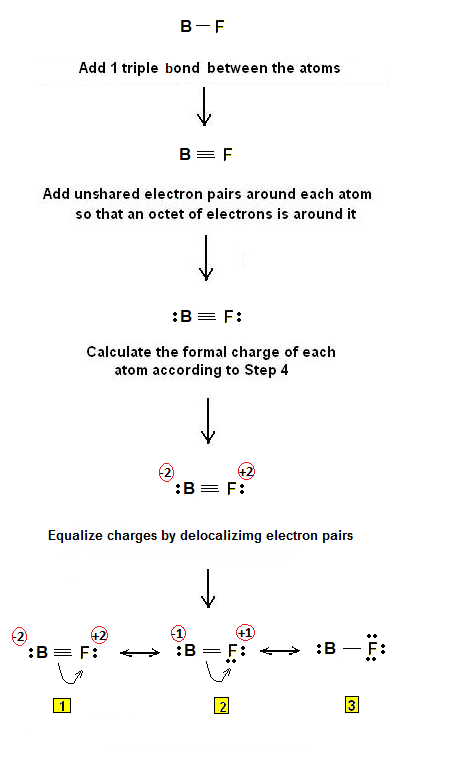
Draw electron dot diagrams for elements. An electron 13, Electron dot diagram for boron.Lewis dot diagram structures show three formal alternatives for describing bonding in boron monofluoride.
BF is unusual in that the dipole moment is inverted with fluorine having a positive charge even though it is the more electronegative element. Originally Answered: How is the Lewis dot structure of boron determined?
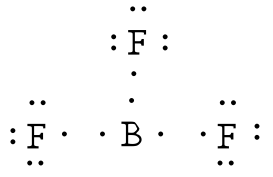
while other elements in the periodic table require 8 electrons to complete their orbitals, boron is a little different. Boron is the only element which requires 6 electrons to complete it’s orbital.
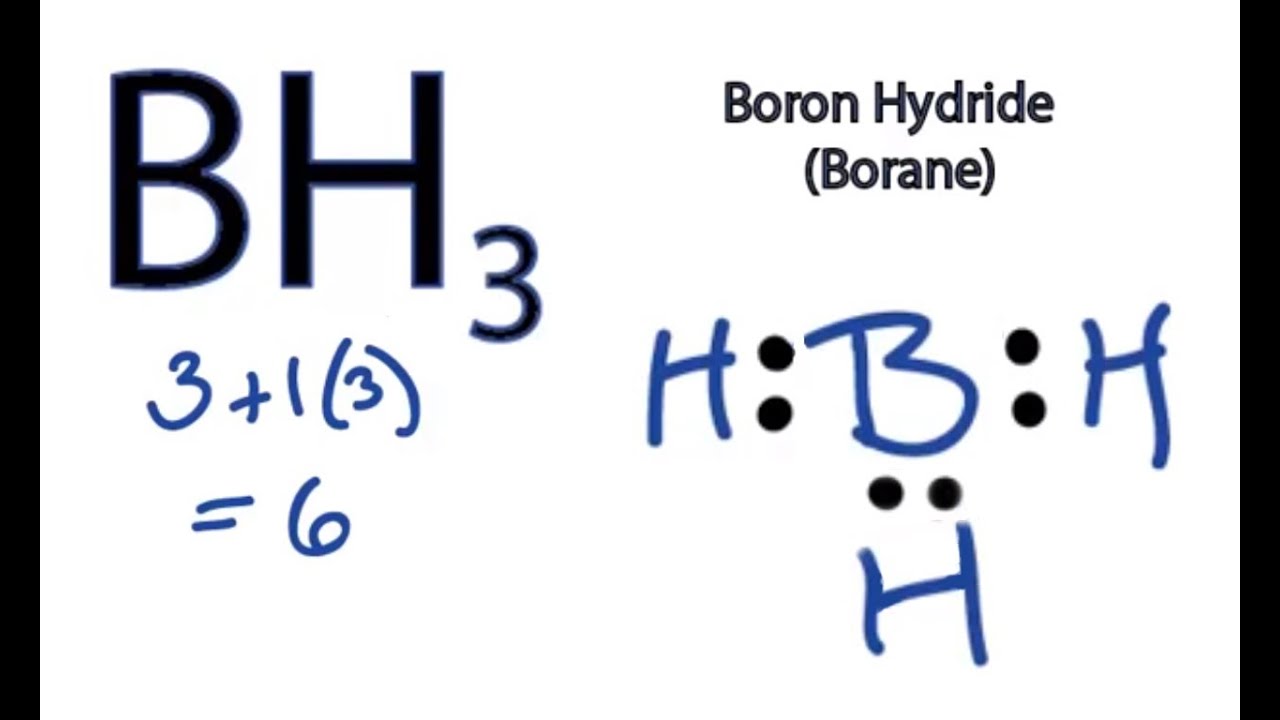
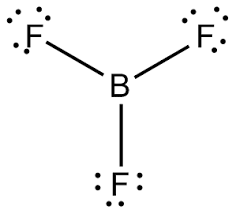
Boron is the central atom surrounded by three iodines with single bonds, and remember boron only need six electrons. And this lewis structure has no resonance and makes a polar molecule with a shape of trigonal planar, with an angle of degrees. Hope this helps!.
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Why is Boron Trifluoride written in two ways in the lewis dot diagram structure? The picture immediately below these sentence makes the most sense to me.
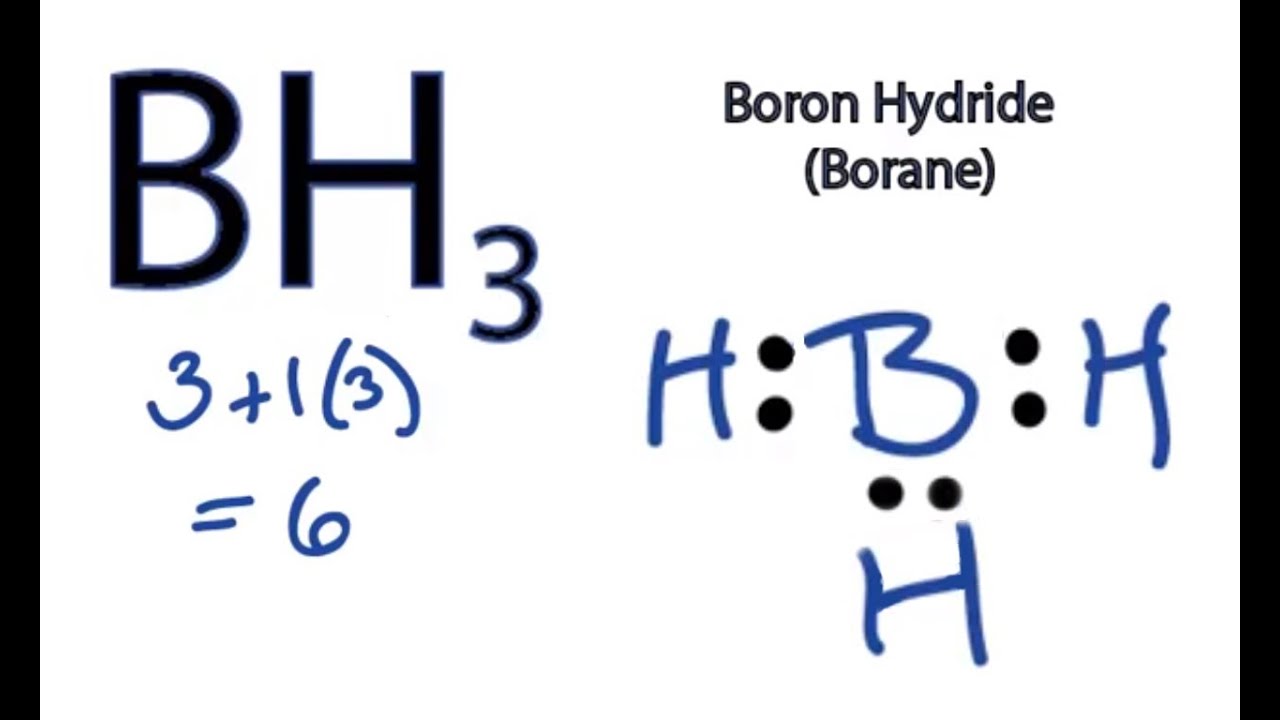
Boron gets an octet of 8 and the respective Fluorides get their octet of 8. Note:Boron disobeys octet rule in Lewis structure. Its a property of it about which we cannot do anything.Lewis StructureBoron monofluoride – Wikipedia
