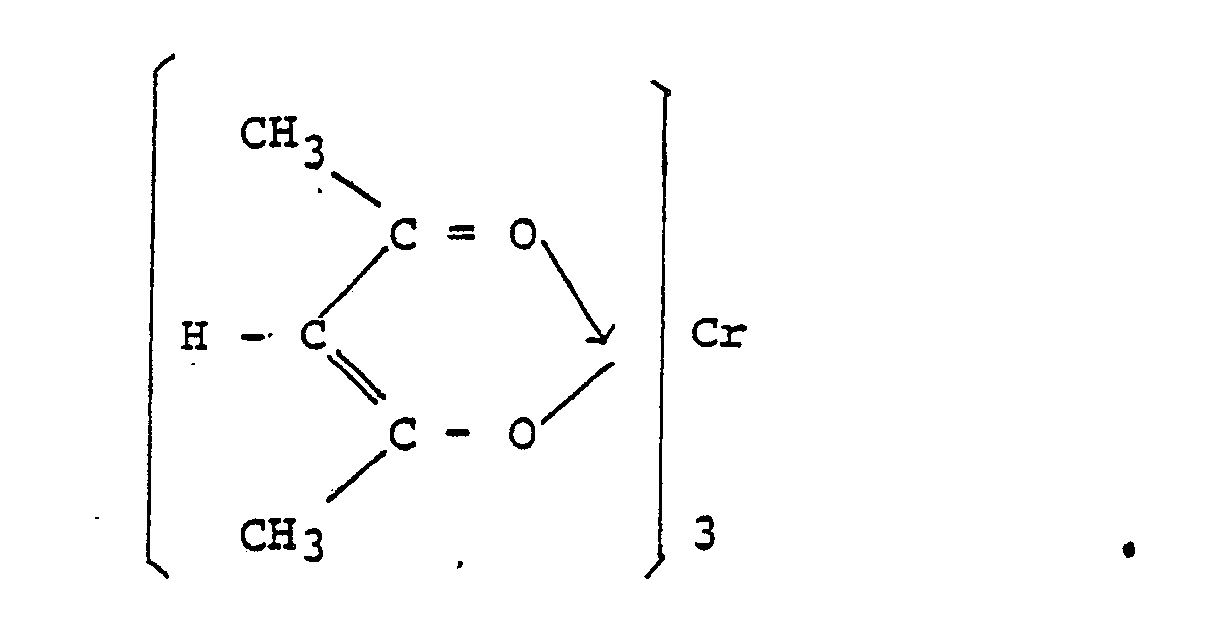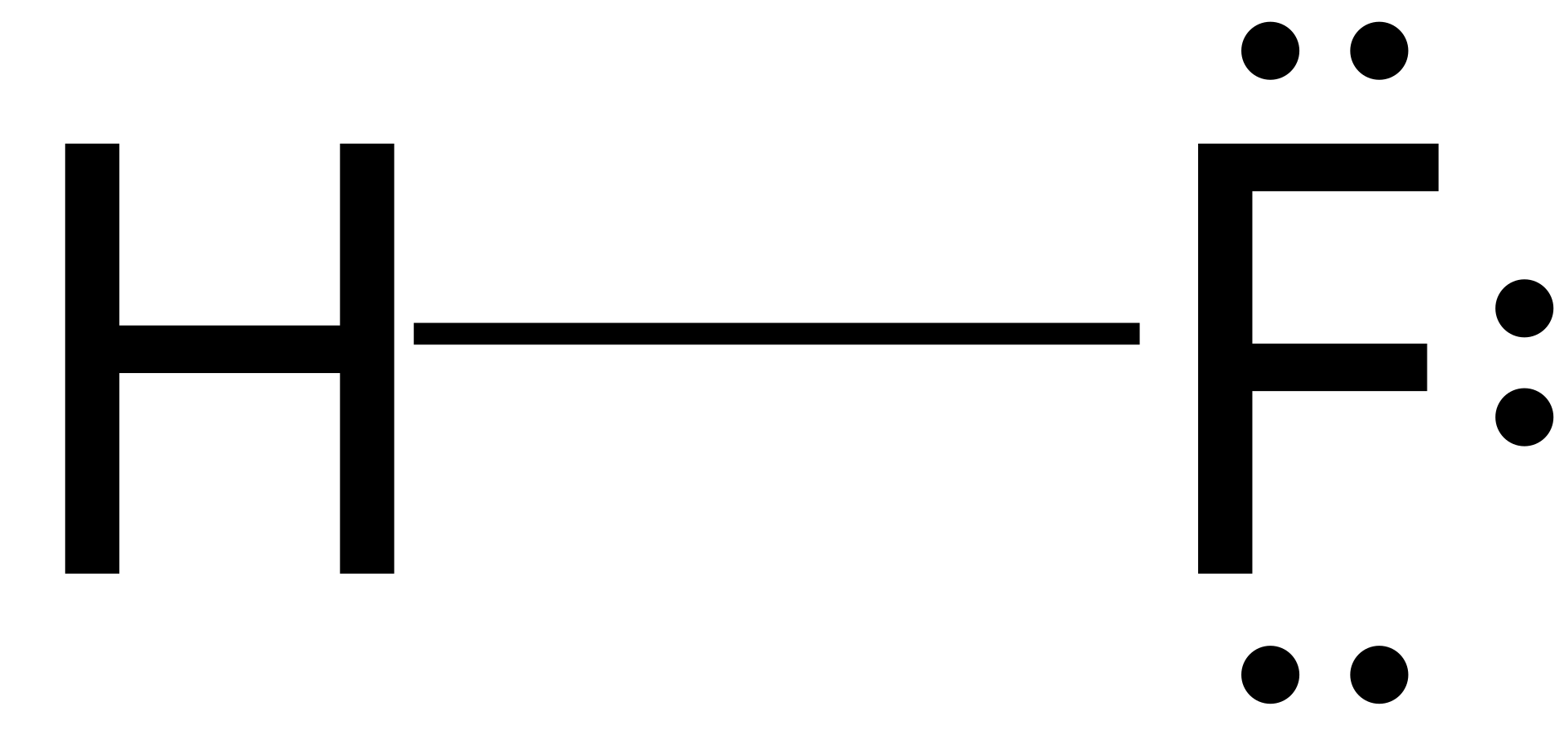
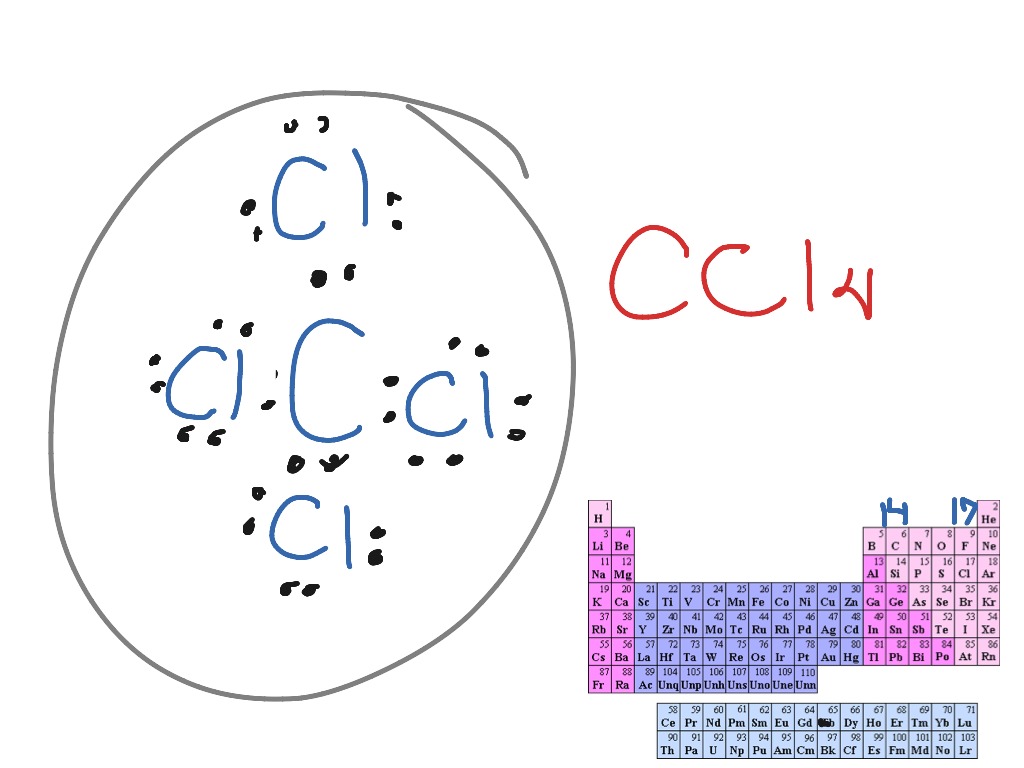
We can use Lewis dot formulas to show covalent bond formation. 1.
H. 2 molecule. +. H.
H. H H..
or H2. H. Cl.
H Cl. + or HCl. 2.
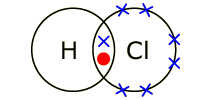
HCl molecule . The left diagram shows a Lewis dot structure of sodium with .. ions, and all of the valence electrons in a HCl molecule are shared between the H and Cl atoms.
Hydrogen’s electron is shown in orange. Chlorine’s valence electron are shown in red.

Thanks to hydrogen’s one electron, the valence shell is. And once they’re dissolved in H2O, they immediately dissociate because HCL is a strong acid with a low pH.
Here’s the Lewis Dot Diagram. Lewis Structure For Hydrogen Chloride Images. Lewis Dot Structures Worksheet – Mr.
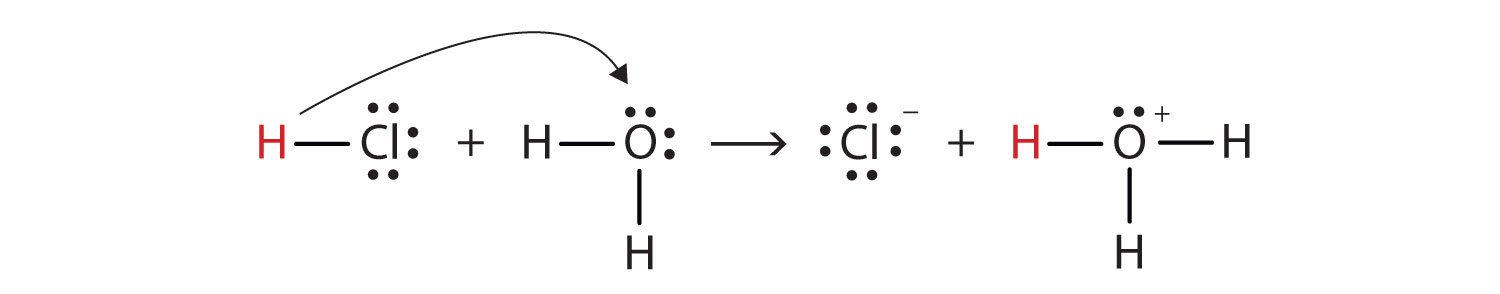
Walsh’s Class Chemical Bonds – Ionic Bonds. Identify the.The Lewis dot structure for HCl has hydrogen’s symbol, H, on the left side, chlorine’s symbol, Cl, on the right side, two dots between the two and an additional six dots around Cl. Hydrogen has a single valence electron, while chlorine has seven valence electrons.
Nov 19, · A step-by-step explanation of how to draw the NaCl Lewis Dot Structure. For the NaCl Lewis structure, calculate the total number of valence electrons for the .
Lewis Dot Formulas of Atoms zLewis dot formulas or Lewis dot representations are a convenient bookkeeping method for tracking valence electrons. •Valence electrons are those electrons that are transferred or involved in chemical bonding. The Lewis Structure for HCl (hydrochloric acid) is one of the easier dot structures to draw.
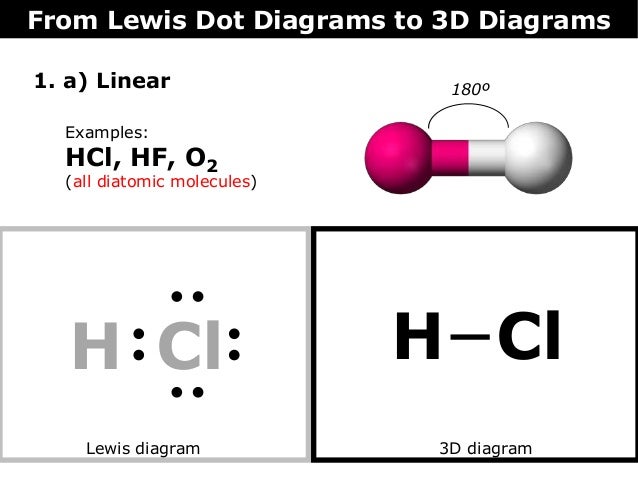
When you draw the structure remember that Hydrogen (H) only needs . The Lewis structure for HCl is a singly bonded H atom and Cl schematron.org the Cl atom there is one pair of dots on each unbonded side.How do you write the electron dot diagram of a hydrogen chloride molecule? | SocraticMolecular Structure: Lewis Dot