It will be on Lewis Dot Structures, specifically ionic compounds!
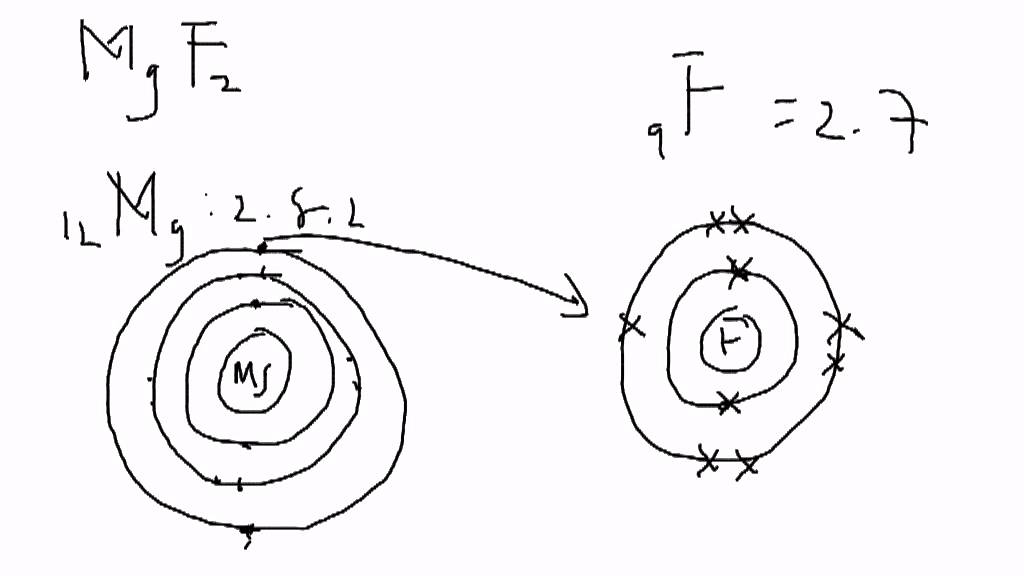
First, let’s go over the Let’s say we have the ionic compound MgF2. That is. Formation of Ionic Bonds We take magnesium fluoride as an example.
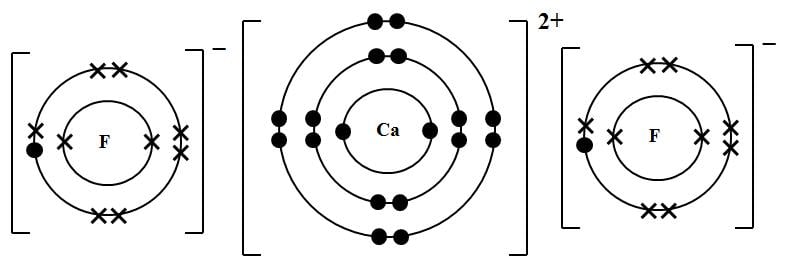
Magnesium Fluoride is an ionic compound. The magnesium atom gives up 2 electrons to form a magnesium ion, Mg2+ Structure of an Ionic Compound.
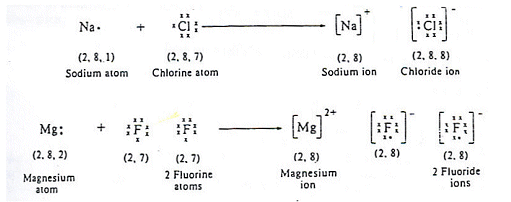
Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound MgF2.
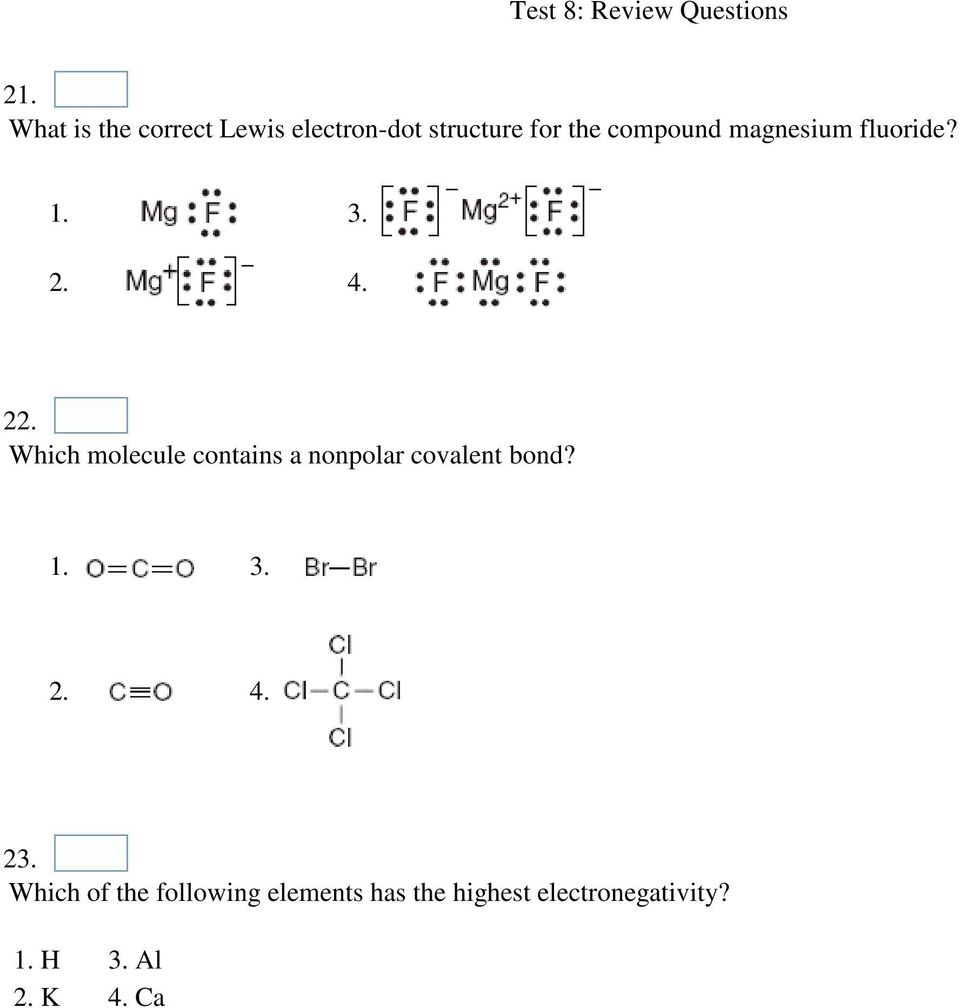
Magnesium fluoride is prepared from magnesium oxide with The structure of the compound is similar to that in rutile. Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluoride?.Dec 18, · Best Answer: Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine, you need 1 Mg atom and 2 F atoms The Mg gives one electrons to each of the F atoms, allowing the Mg to lose its 2 valence electrons and have its (new) outer energy levels fill its octet (8 electrons).Status: Resolved.
After that I draw the Lewis dot structure for Magnesium (Mg). Note: Magnesium is in Group 2 (sometimes called Group II or 2A).

Since it is in Group 2 it will have 2 valence electrons. When you draw the Lewis structure for Magnesium you’ll put two “dots” or .
Name _____ Lewis Dot Structures of Ionic Compounds Date:_____ Chemistry! 1) 2) 3) 4) schematron.org Lewis electron-dot diagram represents an atom in the ground state for a.

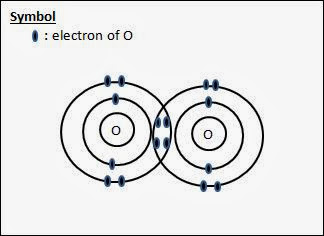
What is the Lewis dot diagram for fluoride? How do Lewis diagrams work in chemistry?
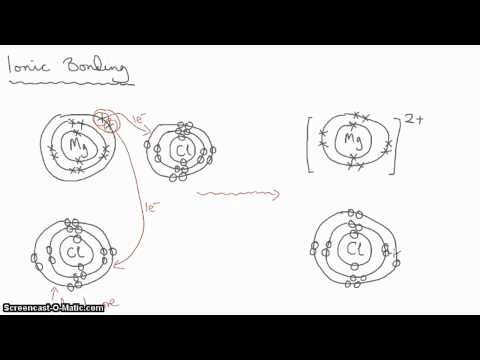
What is the Lewis dot diagram for magnesium? What is the Lewis dot diagram for gold?
What is the Lewis dot diagram for C2H2? What is the Lewis dot diagram for bromine? How is the Lewis dot diagram for neon determined?
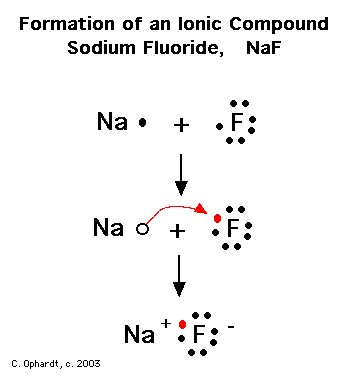
Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound # MgF_2#. Each Florine starts with seven electrons around the atom, combining with the Magnesium atom give the Florine eight electrons around each Florine atom.Lewis Dot Structure (Ionic Bonds) — Teens TeachWhat is the correct lewis electron-dot structure for the compound magnesium fluoride? | Socratic