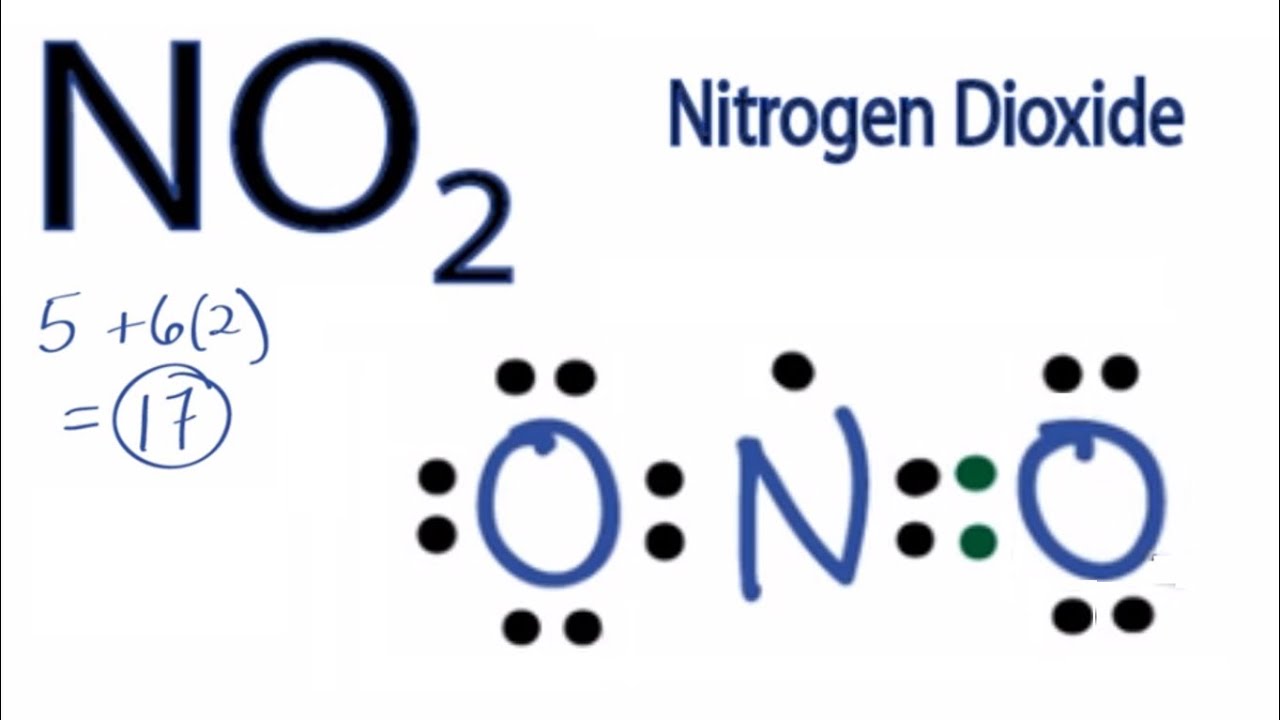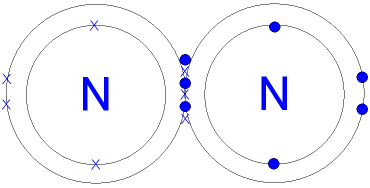
Plucked this image from google.
There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first.
The problem is that there aren’t enough valence electons to give both Nitrogen atoms an octet. You’ll need to use . The Lewis Structure for N2 looks easy at first.

The problem is that there aren’t enough valence electons to give both Nitrogen atoms an octet. You’ll need to use . The Lewis dot structure for any molecule can be found by following a general set of This is derived by following 5 general steps for writing Lewis dot structures.
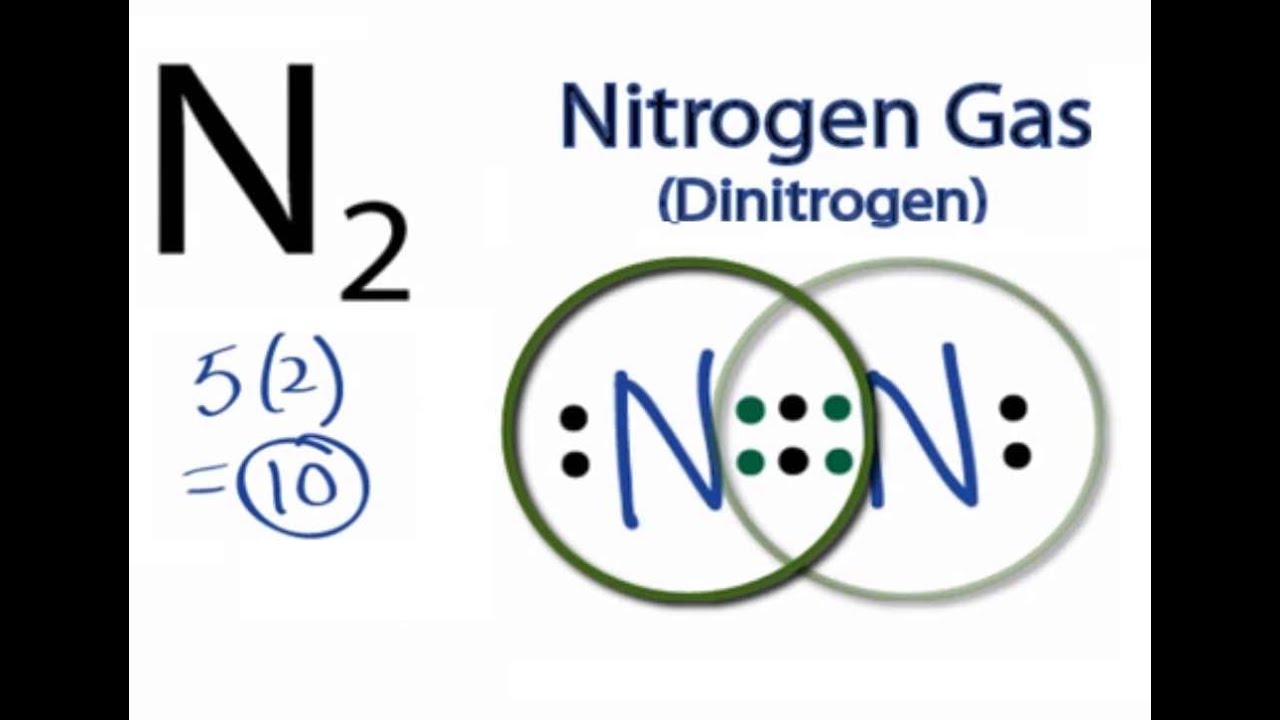
Valence Shell Electron Pair Repulsion Concept. → Predicting the shape/structure of molecules. It was established by Gillespie and Nyholm in the year Here is the electron dot structure for a single #N# atom: The total number of valence electrons between the two #N# atoms is #10 e^-#.
Also, for the structure to be correct, it must follow the octet rule (eight electrons per atom). Hence.
Transcript: For the N2 Lewis structure, we have five valence electrons for Nitrogen–it’s in group 5 or 15 on the periodic table. We have two Nitrogens.
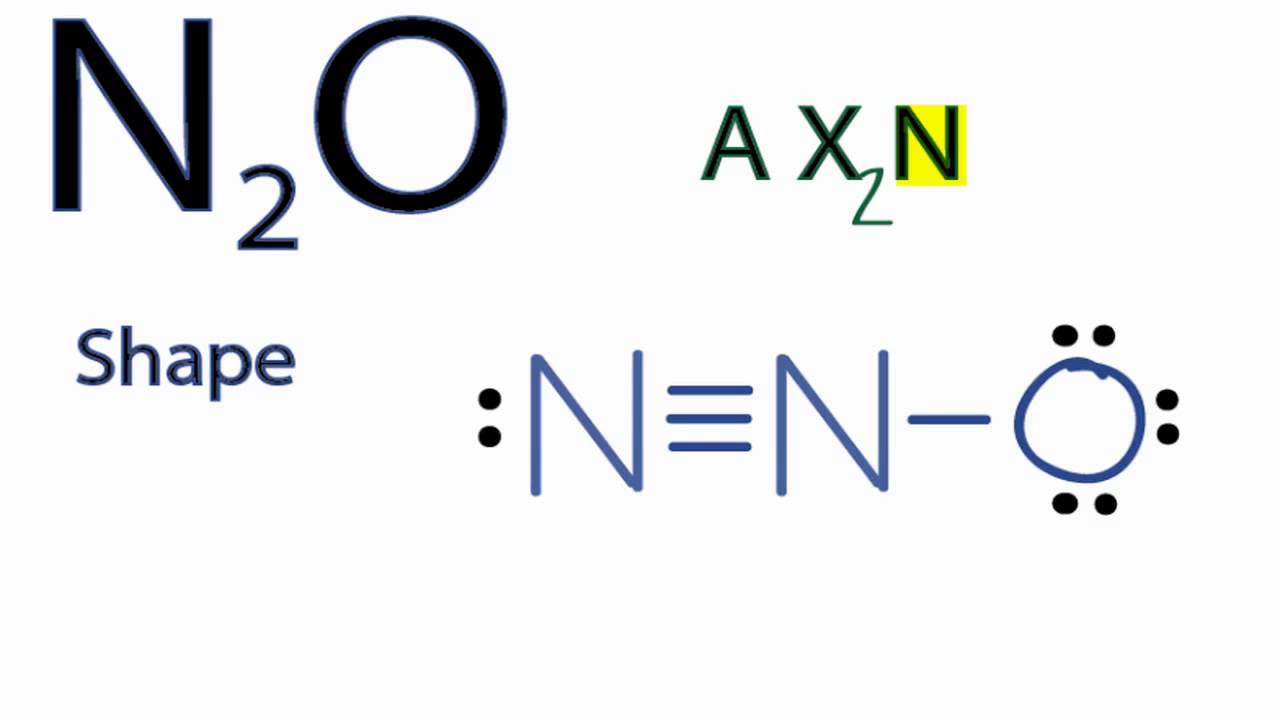
Multiply those together, we have a total of 10 valence electrons for the N2 Lewis structure. We’ll put the two Nitrogens next to each other, and then we’ll put two valence electrons between them to form a chemical bond. Drawing the Lewis Structure for N 2 (Dinitogen or Nitrogen Gas) Nitrogen (N 2) is a commonly tested Lewis structure due to its importance on Earth (about 78% of the Earth’s atomsphere is N 2).
It also is a good example of a molecule with a triple bond. The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter N’s, so that there would be 6 dots total What is the Lewis dot structure for N2 look like?
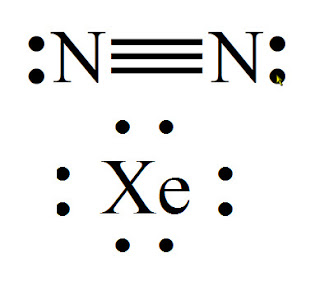
N(triple bond)N and then two dots on each N in which ever spot is open. Share to.Lewis structure – WikipediaWhat is the Lewis structure of N2