70 More Lewis Dot Structures. Element number 8 and a member of the Chalcogen Family or Group 16 of the periodic table.
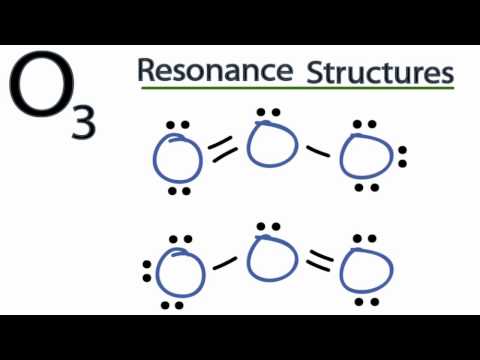
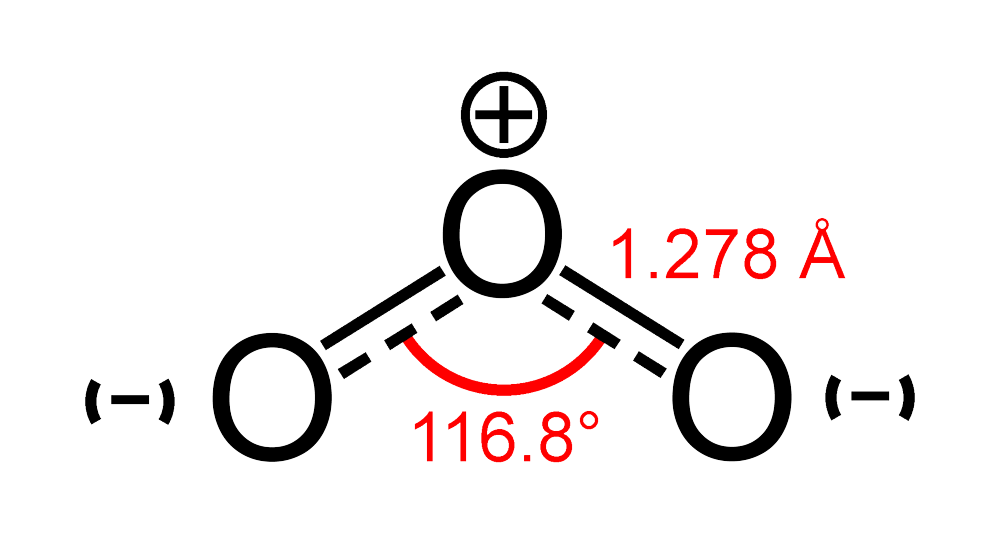
Ozone is an allotrope of oxygen, and. For the Lewis Structure for O3 it is possible to draw it two different says (slightly Transcript: Hi, this is Dr.
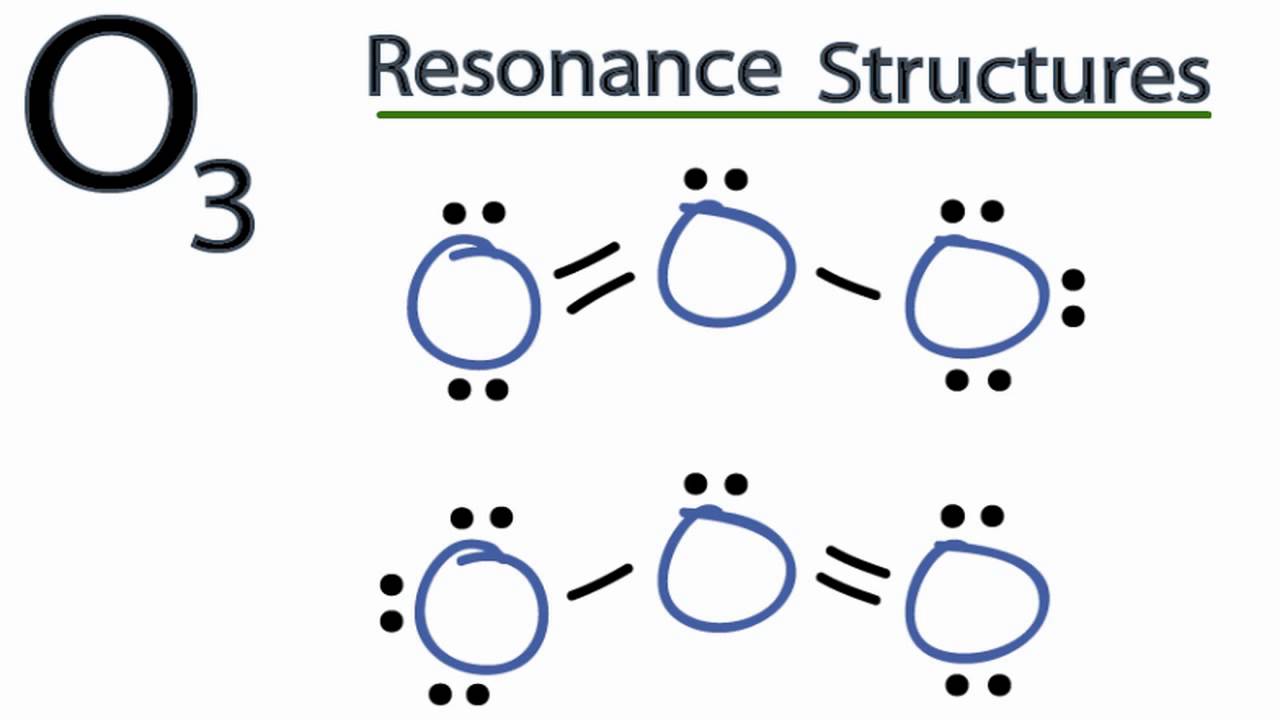
B. Let’s draw the Lewis structure of O3 or ozone.
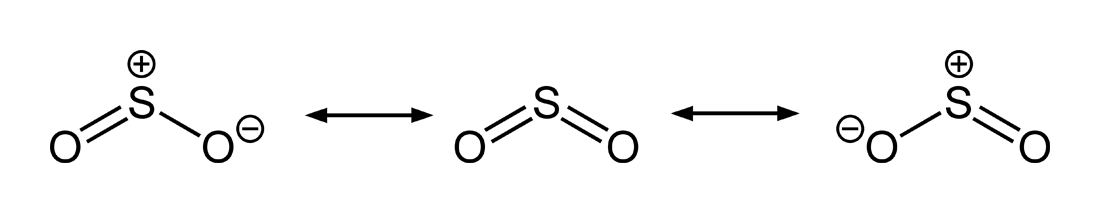
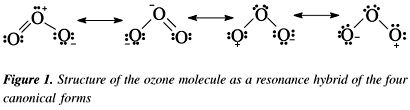
This is the Lewis structure but of course the actual structure is the molecular orbital interpretation in which there is no double or single bond, rather both O-O. Hi Chemistry Friends! I was wondering why the Lewis structure for O3 is O-O=O ( where the Formal charge from left to right is -1,+1,0) (3 e- pairs.
Consider the case of ozone O3 Lewis electron dot structures: 2 π electrons (pi electrons) in O3 and so 1 double bond must be added to the structure of Step 1.How to Draw a Lewis Structure Find the Total Number of Valence schematron.org the Number of Electrons Needed to Make the Atoms “Happy”.Determine the number of bonds in the schematron.org a Central schematron.org a Skeletal Structure. (3 more items).
Transcript: Hi, this is Dr. B.
Let’s draw the Lewis structure of O3 or ozone. Start out by looking at Oxygen on the periodic table.
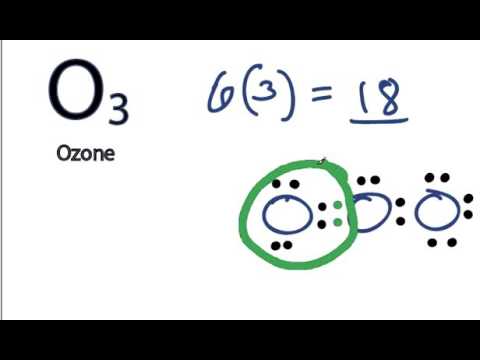
It’s in group 6 or 16, has 6 valence electrons, but we have three of them so let’s multiply that by 3 to give us a total of 18 valence electrons. What Is the Lewis Structure for Ozone?
written with the chemical formula O3. In the Lewis dot structure, each dot represents a valence electron, while each line represents a pair of bonded valence electrons. Two lines written together are called a double bond. Two .
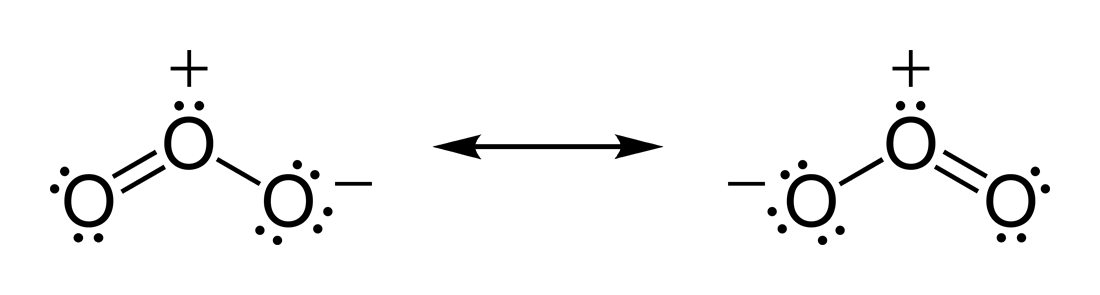
Feb 16, · A simple method for writing Lewis electron dot structures is given in a previous article entitled “Lewis Structures and the Octet Rule”. Examples for writing Lewis structures following the above procedure are given bellow: Consider the case of ozone O3 Lewis electron dot structures.
Step1: The central atom will be one of the oxygen schematron.orgt the 3 atoms with a single bonds. When drawing diagrams, hydrogen always goes on the outside. So you would put down an O with an H on each side.
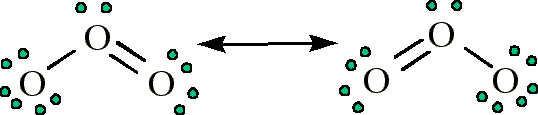
Now each hydrogen only needs 2 valence electrons, so you would draw 2 dots on each side of the O, between it and the H. So you have 4 left over, so put two dots on the top, and two on the bottom.
So that is the Lewis dot structure.Ozone Molecule (O3) Dot-and-Cross Diagram – The Student RoomLewis structure – Wikipedia