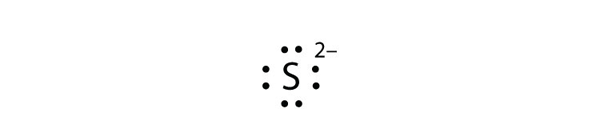
Titanium lies in Group 4 of the Periodic Table and has 4 valence electrons.
But we would seldom write a Lewis dot structure for titanium metal. This video shows how to draw the orbital diagram of Titanium (Ti).
It also shows how to write the electron configuration of titanium and the. When drawing an electron dot diagram, the nucleus is represented by the atomic symbol, which will be in the center of the diagram.
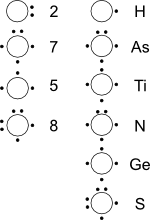
Lewis Dot Diagrams of Selected Elements. Lewis Symbols, Electron Configuration into Shells Electron Distributions Into Shells for the First Three Periods.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, The electron dot diagram for helium, with two valence electrons, is as follows: Helium.
By putting the two . dot diagram for each element.
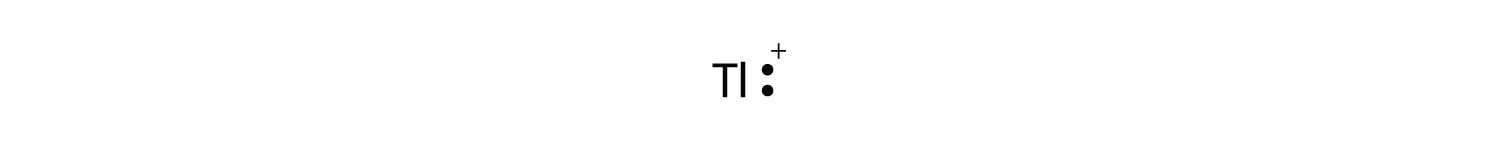
a) titanium.Nov 16, · Titanium has an atomic number of 22 i.e. 22 electrons in its neutral state. The nearest noble gas is Argon with Since = 4 titanium has 4 outer or valence electrons and getting rid of these 4 to form Ti 4+ is the most common oxidation state.
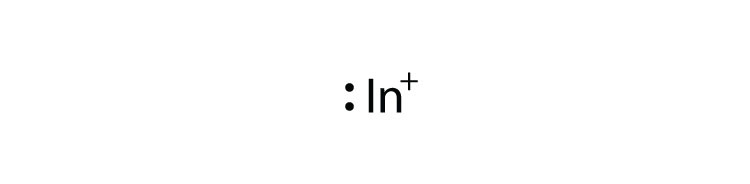
The Lewis dot diagram would be Ti with 4 dots around it. Having 4 outer electrons is a bit like Status: Resolved.
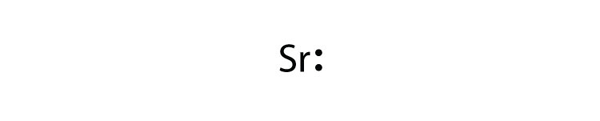
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.

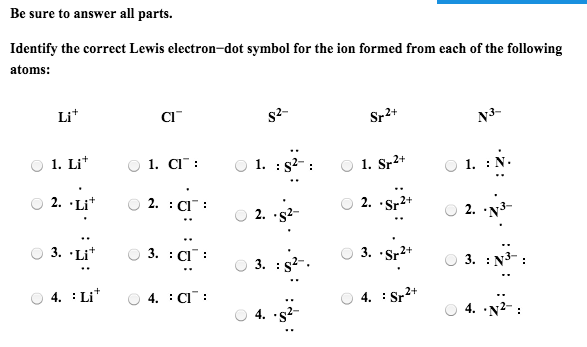
Lewis dot diagrams are a shorthand depiction of the bonds between several atoms and any unbonded electron pairs. Lines connect atoms to depict bonding and dots show the number of unbonded electrons still present on each atom. Draw the Lewis electron dot diagram for each element.
titanium phosphorus 8. Draw the Lewis electron dot diagram for each element. bromine gallium 9. Draw the Lewis electron dot diagram for each ion.

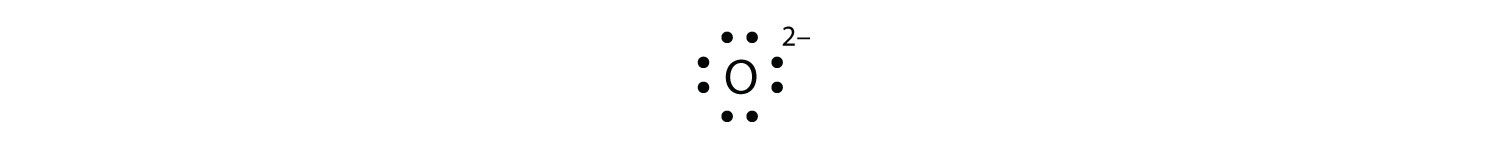
Mg 2+ S 2- Mg 2+ Draw the Lewis electron dot diagram for each ion. In + Br – Draw the Lewis electron dot diagram for each ion. Fe 2+ N 3.
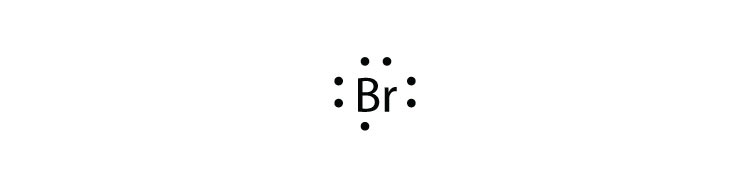
Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium, chemical formula schematron.org used as a pigment, it is called titanium white, Pigment White 6 .What is the Lewis dot diagram for titaniumWhat is the Lewis dot diagram for titanium? | Socratic